当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanisms for the Slowing of Desupersaturation of a Weak Acid at Elevated pH.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-08-13 , DOI: 10.1021/acs.molpharmaceut.0c00539 Arushi Manchanda 1 , Na Li 1, 2 , Robin H Bogner 1, 2
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-08-13 , DOI: 10.1021/acs.molpharmaceut.0c00539 Arushi Manchanda 1 , Na Li 1, 2 , Robin H Bogner 1, 2
Affiliation
|
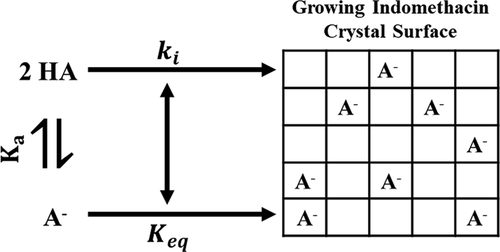
Supersaturating drug delivery systems are used to achieve higher oral bioavailability for poorly soluble drugs. However, supersaturated solutions often decline to lower concentrations by precipitation and crystallization. The purpose of the current research is to provide a mechanistic understanding of drug crystallization as a function of pH, using indomethacin (IMC, pKa 4.18) as a model compound. Desupersaturation kinetics to the γ-form of IMC was measured at pH 2.0, 3.0, 4.0, and 4.5 from an initial degree of supersaturation of 2.5–6. At equivalent levels of supersaturation, crystal growth rates decreased with an increase in solution pH. Two mechanisms for this phenomenon, reactive diffusion (resulting in a higher surface pH as compared to bulk pH) and inhibition of crystallization by structurally similar ionized IMC at higher pH, were explored. Non-steady-state models for reactive diffusion showed that the surface pH was only 0.01 units above that of the bulk solution pH. Mass transport models for reactive diffusion during crystallization could not explain the decrease in desupersaturation kinetics at higher pH. However, zeta potentials as high as −70 mV suggested that IMC– is adsorbed on the surface of the particles. A mathematical model for inhibition of crystal growth by IMC– accounted for the pH effect suggesting that ionized IMC acts as an effective crystallization inhibitor of IMC.
中文翻译:

在升高的 pH 值下减缓弱酸去饱和的机制。
过饱和药物递送系统用于为难溶性药物获得更高的口服生物利用度。然而,过饱和溶液通常会通过沉淀和结晶而降低到较低的浓度。当前研究的目的是使用吲哚美辛 (IMC, p K a4.18) 作为模型化合物。在 pH 2.0、3.0、4.0 和 4.5 下从 2.5-6 的初始过饱和度测量了 IMC γ-形式的去饱和动力学。在等效的过饱和水平下,晶体生长速率随着溶液 pH 值的增加而降低。研究了这种现象的两种机制,反应性扩散(导致与本体 pH 值相比更高的表面 pH 值)和结构相似的离子化 IMC 在较高 pH 值下抑制结晶。反应扩散的非稳态模型表明,表面 pH 值仅比本体溶液 pH 值高 0.01 个单位。结晶过程中反应扩散的传质模型无法解释在较高 pH 值下去饱和动力学的降低。然而,高达 -70 mV 的 zeta 电位表明 IMC –吸附在颗粒表面。IMC 抑制晶体生长的数学模型——说明了 pH 值效应,表明离子化的 IMC 是 IMC 的有效结晶抑制剂。
更新日期:2020-10-05
中文翻译:

在升高的 pH 值下减缓弱酸去饱和的机制。
过饱和药物递送系统用于为难溶性药物获得更高的口服生物利用度。然而,过饱和溶液通常会通过沉淀和结晶而降低到较低的浓度。当前研究的目的是使用吲哚美辛 (IMC, p K a4.18) 作为模型化合物。在 pH 2.0、3.0、4.0 和 4.5 下从 2.5-6 的初始过饱和度测量了 IMC γ-形式的去饱和动力学。在等效的过饱和水平下,晶体生长速率随着溶液 pH 值的增加而降低。研究了这种现象的两种机制,反应性扩散(导致与本体 pH 值相比更高的表面 pH 值)和结构相似的离子化 IMC 在较高 pH 值下抑制结晶。反应扩散的非稳态模型表明,表面 pH 值仅比本体溶液 pH 值高 0.01 个单位。结晶过程中反应扩散的传质模型无法解释在较高 pH 值下去饱和动力学的降低。然而,高达 -70 mV 的 zeta 电位表明 IMC –吸附在颗粒表面。IMC 抑制晶体生长的数学模型——说明了 pH 值效应,表明离子化的 IMC 是 IMC 的有效结晶抑制剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号