Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecularly Imprinted Nanogels Possessing Dansylamide Interaction Sites for Controlling Protein Corona In Situ by Cloaking Intrinsic Human Serum Albumin.
Langmuir ( IF 3.7 ) Pub Date : 2020-08-14 , DOI: 10.1021/acs.langmuir.0c00927 Takahiro Morishita 1 , Aoi Yoshida 1 , Natsuki Hayakawa 1 , Kentaro Kiguchi 1 , Chehasan Cheubong 1, 2 , Hirobumi Sunayama 1 , Yukiya Kitayama 3 , Toshifumi Takeuchi 1, 4
Langmuir ( IF 3.7 ) Pub Date : 2020-08-14 , DOI: 10.1021/acs.langmuir.0c00927 Takahiro Morishita 1 , Aoi Yoshida 1 , Natsuki Hayakawa 1 , Kentaro Kiguchi 1 , Chehasan Cheubong 1, 2 , Hirobumi Sunayama 1 , Yukiya Kitayama 3 , Toshifumi Takeuchi 1, 4
Affiliation
|
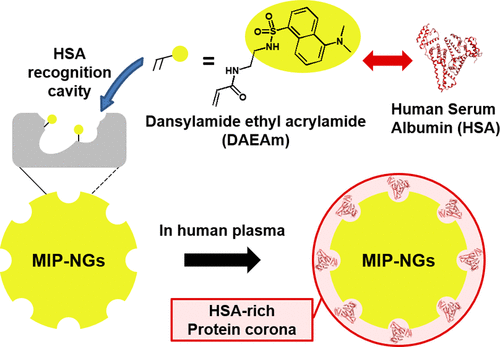
Nanomaterials have become increasingly promising for biomedical applications owing to their specific biological characteristics. As drug delivery vehicles, nanomaterials have to circulate in the bloodstream to deliver the encapsulated components to the target tissues. Protein corona regulation is one of the promising approaches that gives stealth capability to avoid immune response. The aim of this study was to develop molecularly imprinted polymer nanogels (MIP-NGs) capable of protein corona regulation, using intrinsic human serum albumin (HSA) and with a functional monomer, dansylamide ethyl acrylamide (DAEAm), the dansylamide group serving as a ligand for HSA. The recognition capability of HSA for MIP-NGs was investigated by isothermal titration calorimetry (ITC). The affinity of the MIP-NGs prepared with DAEAm was then compared to that of the reference MIP-NGs prepared with pyrrolidyl acrylate developed in our previous study. Furthermore, we demonstrated that the concurrent use of these two different functional monomers for molecular imprinting was further effective to construct high-affinity recognition nanocavities for HSA and to form HSA-rich protein corona in the human plasma owing to the different interaction modes of the monomers. We believe that the molecular imprinting strategy developed through the use of ligand-based functional monomer is an effective strategy to create artificial molecular recognition materials.
中文翻译:

分子印迹纳米凝胶具有丹磺酰胺相互作用位点,通过掩盖人体内固有的血清白蛋白来控制蛋白电晕。
纳米材料由于其特定的生物学特性而在生物医学应用中变得越来越有希望。作为药物输送工具,纳米材料必须在血液中循环以将包封的组分输送至目标组织。蛋白质电晕调节是一种有前途的方法,它具有隐身能力来避免免疫反应。这项研究的目的是使用内在的人血清白蛋白(HSA)和功能性单体丹磺酰胺乙基丙烯酰胺(DAEAm)来开发能够进行蛋白质电晕调节的分子印迹聚合物纳米凝胶(MIP-NGs),该丹磺酰胺基团作为HSA的配体。HSA对MIP-NGs的识别能力通过等温滴定热法(ITC)进行了研究。然后将DAEAm制备的MIP-NGs与我们先前研究开发的丙烯酸丙烯酸吡咯烷基酯制备的参比MIP-NGs的亲和力进行了比较。此外,我们证明了同时使用这两种不同的功能单体进行分子印迹可进一步有效地构建高亲和力识别HSA的纳米腔,并由于单体的相互作用方式不同而在人血浆中形成富含HSA的蛋白质电晕。我们认为,通过使用基于配体的功能单体开发的分子印迹策略是创建人工分子识别材料的有效策略。我们证明,同时使用这两种不同的功能单体进行分子印迹,由于单体的相互作用方式不同,因此可以更有效地为人血浆构建高亲和力识别纳米腔并在人血浆中形成富含HSA的蛋白质电晕。我们认为,通过使用基于配体的功能单体开发的分子印迹策略是创建人工分子识别材料的有效策略。我们证明,同时使用这两种不同的功能单体进行分子印迹,由于单体的相互作用方式不同,因此可以更有效地为人血浆构建高亲和力识别纳米腔并在人血浆中形成富含HSA的蛋白质电晕。我们认为,通过使用基于配体的功能单体开发的分子印迹策略是创建人工分子识别材料的有效策略。
更新日期:2020-09-15
中文翻译:

分子印迹纳米凝胶具有丹磺酰胺相互作用位点,通过掩盖人体内固有的血清白蛋白来控制蛋白电晕。
纳米材料由于其特定的生物学特性而在生物医学应用中变得越来越有希望。作为药物输送工具,纳米材料必须在血液中循环以将包封的组分输送至目标组织。蛋白质电晕调节是一种有前途的方法,它具有隐身能力来避免免疫反应。这项研究的目的是使用内在的人血清白蛋白(HSA)和功能性单体丹磺酰胺乙基丙烯酰胺(DAEAm)来开发能够进行蛋白质电晕调节的分子印迹聚合物纳米凝胶(MIP-NGs),该丹磺酰胺基团作为HSA的配体。HSA对MIP-NGs的识别能力通过等温滴定热法(ITC)进行了研究。然后将DAEAm制备的MIP-NGs与我们先前研究开发的丙烯酸丙烯酸吡咯烷基酯制备的参比MIP-NGs的亲和力进行了比较。此外,我们证明了同时使用这两种不同的功能单体进行分子印迹可进一步有效地构建高亲和力识别HSA的纳米腔,并由于单体的相互作用方式不同而在人血浆中形成富含HSA的蛋白质电晕。我们认为,通过使用基于配体的功能单体开发的分子印迹策略是创建人工分子识别材料的有效策略。我们证明,同时使用这两种不同的功能单体进行分子印迹,由于单体的相互作用方式不同,因此可以更有效地为人血浆构建高亲和力识别纳米腔并在人血浆中形成富含HSA的蛋白质电晕。我们认为,通过使用基于配体的功能单体开发的分子印迹策略是创建人工分子识别材料的有效策略。我们证明,同时使用这两种不同的功能单体进行分子印迹,由于单体的相互作用方式不同,因此可以更有效地为人血浆构建高亲和力识别纳米腔并在人血浆中形成富含HSA的蛋白质电晕。我们认为,通过使用基于配体的功能单体开发的分子印迹策略是创建人工分子识别材料的有效策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号