当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Three Reversible Redox States of Thiolate-Bridged Dirhodium Complexes without Metal-Metal Bonds
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-08-13 , DOI: 10.1021/jacs.0c06205 Ryan P Coll 1 , Kim R Dunbar 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-08-13 , DOI: 10.1021/jacs.0c06205 Ryan P Coll 1 , Kim R Dunbar 1
Affiliation
|
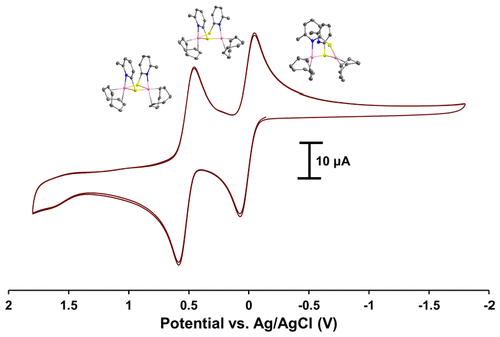
An unusual dinuclear rhodium complex with the anionic 2-mercapto-6-methylpyridinate (mmp) bridging ligand is reported which is capable of undergoing significant variations in its structural and coordination environments as a result of two reversible redox events at accessible potentials (E1/2 = 0.014, 0.52 V vs. Ag/AgCl). The large degree of separation between these redox states (ΔE = 0.51 V, KC = 4.17x108) allows for the chemical isolation of three distinct complexes 1, 2, and 3, in which the oxidation states of each Rh center are described as Rh2I,I, Rh2I,II, and Rh2II,II, respectively, and whose structures were elucidated by single crystal X-ray diffraction studies. Complex 2 is an unprecedented type of mixed valence dirhodium species whose EPR spectrum revealed a delocalization of the unpaired electron through the thiolate bridging ligand. Intervalence charge transfer (IVCT) occurs between the Rh centers as evidenced by a broad absorption in the near-infrared region (λmax¬=1187 nm). The structure of 3 is quite rare in that it lacks the typical RhII-RhII σ bond but significant orbital overlap between the Rh 4dz2 and S 3pz orbitals results in strong antiferromagnetic coupling (computed J = -1516.9 cm-1). Complex 3 also absorbs low energy light (λmax¬ = 779 nm) and spectroscopic and magnetic measurements are supported by DFT methods which further elucidate the nature of the ground state energies, frontier orbital characters, excitated state transitions, and the presence of weak Rh-Rh NBO interactions.
中文翻译:

无金属-金属键的硫醇盐-桥接铑配合物的三种可逆氧化还原态
据报道,一种具有阴离子 2-巯基-6-甲基吡啶 (mmp) 桥连配体的不寻常双核铑配合物能够在其结构和配位环境中发生显着变化,这是由于在可及电位 (E1/2) 下发生的两个可逆氧化还原事件= 0.014, 0.52 V vs. Ag/AgCl)。这些氧化还原状态(ΔE = 0.51 V,KC = 4.17x108)之间的大程度分离允许对三个不同的复合物 1、2 和 3 进行化学分离,其中每个 Rh 中心的氧化态被描述为 Rh2I, I、Rh2I,II 和 Rh2II,II 分别通过单晶 X 射线衍射研究阐明了其结构。复合物 2 是一种前所未有的混合价二铑物种,其 EPR 光谱显示未配对电子通过硫醇桥配体离域。间隔电荷转移 (IVCT) 发生在 Rh 中心之间,如近红外区域 (λmax = 1187 nm) 中的广泛吸收所证明的那样。3 的结构非常罕见,因为它缺乏典型的 RhII-RhII σ 键,但 Rh 4dz2 和 S 3pz 轨道之间的显着轨道重叠导致强烈的反铁磁耦合(计算 J = -1516.9 cm-1)。复合物 3 还吸收低能量光(λmax= 779 nm),光谱和磁测量得到 DFT 方法的支持,这些方法进一步阐明了基态能量的性质、前沿轨道特征、激发态跃迁和弱 Rh-的存在。 Rh NBO 相互作用。3 的结构非常罕见,因为它缺乏典型的 RhII-RhII σ 键,但 Rh 4dz2 和 S 3pz 轨道之间的显着轨道重叠导致强烈的反铁磁耦合(计算 J = -1516.9 cm-1)。复合物 3 还吸收低能量光 (λmax= 779 nm),光谱和磁测量得到 DFT 方法的支持,这些方法进一步阐明了基态能量的性质、前沿轨道特征、激发态跃迁和弱 Rh-的存在。 Rh NBO 相互作用。3 的结构非常罕见,因为它缺乏典型的 RhII-RhII σ 键,但 Rh 4dz2 和 S 3pz 轨道之间的显着轨道重叠导致强烈的反铁磁耦合(计算 J = -1516.9 cm-1)。复合物 3 还吸收低能量光 (λmax= 779 nm),光谱和磁测量得到 DFT 方法的支持,这些方法进一步阐明了基态能量的性质、前沿轨道特征、激发态跃迁和弱 Rh-的存在。 Rh NBO 相互作用。
更新日期:2020-08-13
中文翻译:

无金属-金属键的硫醇盐-桥接铑配合物的三种可逆氧化还原态
据报道,一种具有阴离子 2-巯基-6-甲基吡啶 (mmp) 桥连配体的不寻常双核铑配合物能够在其结构和配位环境中发生显着变化,这是由于在可及电位 (E1/2) 下发生的两个可逆氧化还原事件= 0.014, 0.52 V vs. Ag/AgCl)。这些氧化还原状态(ΔE = 0.51 V,KC = 4.17x108)之间的大程度分离允许对三个不同的复合物 1、2 和 3 进行化学分离,其中每个 Rh 中心的氧化态被描述为 Rh2I, I、Rh2I,II 和 Rh2II,II 分别通过单晶 X 射线衍射研究阐明了其结构。复合物 2 是一种前所未有的混合价二铑物种,其 EPR 光谱显示未配对电子通过硫醇桥配体离域。间隔电荷转移 (IVCT) 发生在 Rh 中心之间,如近红外区域 (λmax = 1187 nm) 中的广泛吸收所证明的那样。3 的结构非常罕见,因为它缺乏典型的 RhII-RhII σ 键,但 Rh 4dz2 和 S 3pz 轨道之间的显着轨道重叠导致强烈的反铁磁耦合(计算 J = -1516.9 cm-1)。复合物 3 还吸收低能量光(λmax= 779 nm),光谱和磁测量得到 DFT 方法的支持,这些方法进一步阐明了基态能量的性质、前沿轨道特征、激发态跃迁和弱 Rh-的存在。 Rh NBO 相互作用。3 的结构非常罕见,因为它缺乏典型的 RhII-RhII σ 键,但 Rh 4dz2 和 S 3pz 轨道之间的显着轨道重叠导致强烈的反铁磁耦合(计算 J = -1516.9 cm-1)。复合物 3 还吸收低能量光 (λmax= 779 nm),光谱和磁测量得到 DFT 方法的支持,这些方法进一步阐明了基态能量的性质、前沿轨道特征、激发态跃迁和弱 Rh-的存在。 Rh NBO 相互作用。3 的结构非常罕见,因为它缺乏典型的 RhII-RhII σ 键,但 Rh 4dz2 和 S 3pz 轨道之间的显着轨道重叠导致强烈的反铁磁耦合(计算 J = -1516.9 cm-1)。复合物 3 还吸收低能量光 (λmax= 779 nm),光谱和磁测量得到 DFT 方法的支持,这些方法进一步阐明了基态能量的性质、前沿轨道特征、激发态跃迁和弱 Rh-的存在。 Rh NBO 相互作用。







































 京公网安备 11010802027423号
京公网安备 11010802027423号