当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Peptide Fibrillar Assemblies Exhibit Membranolytic Effects and Antimetastatic Activity on Lung Cancer Cells.
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-08-13 , DOI: 10.1021/acs.biomac.0c00911 Yu-Fon Chen,Chien-Hsiang Chang,Ming-Wei Hsu,Ho-Min Chang,Yi-Cheng Chen,Yi-Sheng Jiang,Jeng-Shiung Jan
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-08-13 , DOI: 10.1021/acs.biomac.0c00911 Yu-Fon Chen,Chien-Hsiang Chang,Ming-Wei Hsu,Ho-Min Chang,Yi-Cheng Chen,Yi-Sheng Jiang,Jeng-Shiung Jan
|
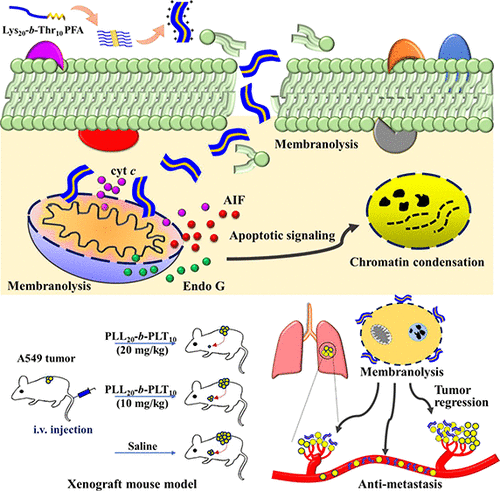
Cancer metastasis is a central oncology concern that worsens patient conditions and increases mortality in a short period of time. During metastatic events, mitochondria undergo specific physiological alterations that have emerged as notable therapeutic targets to counter cancer progression. In this study, we use drug-free, cationic peptide fibrillar assemblies (PFAs) formed by poly(L-Lysine)-block-poly(L-Threonine) (Lys-b-Thr) to target mitochondria. These PFAs interact with cellular and mitochondrial membranes via electrostatic interactions, resulting in membranolysis. Charge repulsion and hydrogen-bonding interactions exerted by Lys and Thr segments dictate the packing of the peptides and enable the PFAs to display enhanced membranolytic activity toward cancer cells. Cytochrome c (cyt c), endonuclease G, and apoptosis-inducing factor were released from mitochondria after treatment of lung cancer cells, subsequently inducing caspase-dependent and caspase-independent apoptotic pathways. A metastatic xenograft mouse model was used to show how the PFAs significantly suppressed lung metastasis and inhibited tumor growth, while avoiding significant body weight loss and mortality. Antimetastatic activities of PFAs are also demonstrated by in vitro inhibition of lung cancer cell migration and clonogenesis. Our results imply that the cationic PFAs achieved the intended and targeted mitochondrial damage, providing an efficient antimetastatic therapy.
中文翻译:

肽原纤维集合体对肺癌细胞具有膜分解作用和抗转移活性。
癌症转移是肿瘤学的中心问题,它会使患者的病情恶化并在短时间内增加死亡率。在转移事件中,线粒体经历了特定的生理变化,这些变化已成为对抗癌症进展的显着治疗靶标。在这项研究中,我们使用无毒,阳离子肽原纤维组件(非疫区)由聚形成(L-赖氨酸) -嵌段-聚(L-苏氨酸)(赖氨酸- b -Thr)到目标线粒体。这些PFA通过静电相互作用与细胞膜和线粒体膜相互作用,从而导致膜溶解。Lys和Thr片段所产生的电荷排斥和氢键相互作用决定了肽的堆积,并使PFA对癌细胞表现出增强的膜分解活性。细胞色素处理肺癌细胞后,c(cyt c),核酸内切酶G和凋亡诱导因子从线粒体中释放出来,随后诱导caspase依赖性和caspase依赖性凋亡途径。使用转移性异种移植小鼠模型显示PFA如何显着抑制肺转移并抑制肿瘤生长,同时避免显着的体重减轻和死亡率。通过体外抑制肺癌细胞迁移和克隆形成也证明了PFA的抗转移活性。我们的结果表明,阳离子PFA达到了预期的和靶向的线粒体损伤,提供了有效的抗转移疗法。
更新日期:2020-09-14
中文翻译:

肽原纤维集合体对肺癌细胞具有膜分解作用和抗转移活性。
癌症转移是肿瘤学的中心问题,它会使患者的病情恶化并在短时间内增加死亡率。在转移事件中,线粒体经历了特定的生理变化,这些变化已成为对抗癌症进展的显着治疗靶标。在这项研究中,我们使用无毒,阳离子肽原纤维组件(非疫区)由聚形成(L-赖氨酸) -嵌段-聚(L-苏氨酸)(赖氨酸- b -Thr)到目标线粒体。这些PFA通过静电相互作用与细胞膜和线粒体膜相互作用,从而导致膜溶解。Lys和Thr片段所产生的电荷排斥和氢键相互作用决定了肽的堆积,并使PFA对癌细胞表现出增强的膜分解活性。细胞色素处理肺癌细胞后,c(cyt c),核酸内切酶G和凋亡诱导因子从线粒体中释放出来,随后诱导caspase依赖性和caspase依赖性凋亡途径。使用转移性异种移植小鼠模型显示PFA如何显着抑制肺转移并抑制肿瘤生长,同时避免显着的体重减轻和死亡率。通过体外抑制肺癌细胞迁移和克隆形成也证明了PFA的抗转移活性。我们的结果表明,阳离子PFA达到了预期的和靶向的线粒体损伤,提供了有效的抗转移疗法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号