当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Significance of Individual Domains of ClpL: A Novel Chaperone from Streptococcus mutans.
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-14 , DOI: 10.1021/acs.biochem.0c00544 Biswanath Jana 1 , Indranil Biswas 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-14 , DOI: 10.1021/acs.biochem.0c00544 Biswanath Jana 1 , Indranil Biswas 1
Affiliation
|
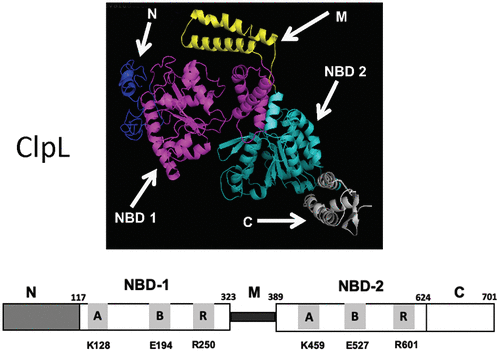
ClpL is a member of the HSP100 family of AAA+ chaperones that is widely present in Gram-positive but surprisingly absent in Gram-negative bacteria. ClpL is involved in various cellular processes, including stress tolerance response, long-term survival, virulence, and antibiotic resistance. ClpL is poorly characterized, and its molecular mechanisms of chaperone activity are largely unclear. Here, we biochemically characterized the ClpL protein from Streptococcus mutans, a dental pathogen, to understand its biological functions. ClpL harbors five domains: N-domain, two nucleotide binding domains (NBD-1 and NBD-2), M-domain, and C-domain. NBD-1 and NBD-2 contain distinct Walker A and B motifs for ATP binding and hydrolysis, respectively. We found that ClpL predominantly exists as a trimer in solution; however, upon ATP binding, it rapidly forms a hexameric structure. To study structure–function activity, we constructed several substitution and deletion mutants. We found that mutations in the Walker A and B motifs interfered with ATP hydrolysis and oligomerization. Similarly, deletions of N-, M-, and C-domains abolished both ATPase activity and oligomerization. Because we previously found that ClpL acts as a chaperone, we analyzed the chaperone activity. Surprisingly, we found that the NBD-2 mutants did not display any chaperone activity, indicating that ATP binding and hydrolysis by NBD-2 are essential for the chaperone. However, NBD-1 mutants showed chaperone activities, but the activities were variable depending on the nature of the mutations. Our results indicate that unlike other HSP100 family chaperones, ClpL is a novel chaperone that does not require any additional secondary chaperones for its activity.
中文翻译:

ClpL 单个域的意义:来自变形链球菌的新型伴侣。
ClpL 是 AAA+ 分子伴侣的 HSP100 家族成员,广泛存在于革兰氏阳性菌中,但出人意料地不存在于革兰氏阴性菌中。ClpL 参与各种细胞过程,包括应激耐受反应、长期存活、毒力和抗生素抗性。ClpL 的特征很差,其分子伴侣活性的分子机制尚不清楚。在这里,我们对来自变形链球菌的 ClpL 蛋白进行了生化表征,一种牙科病原体,以了解其生物学功能。ClpL 包含五个域:N 域、两个核苷酸结合域(NBD-1 和 NBD-2)、M 域和 C 域。NBD-1 和 NBD-2 分别包含不同的 Walker A 和 B 基序,用于 ATP 结合和水解。我们发现 ClpL 在溶液中主要以三聚体形式存在;然而,在与 ATP 结合后,它会迅速形成六聚体结构。为了研究结构-功能活性,我们构建了几个替换和缺失突变体。我们发现 Walker A 和 B 基序中的突变干扰了 ATP 水解和寡聚化。同样,N-、M-和C-结构域的缺失消除了ATPase 活性和寡聚化。因为我们之前发现 ClpL 充当伴侣,所以我们分析了伴侣活动。出奇,我们发现 NBD-2 突变体没有显示任何分子伴侣活性,表明 NBD-2 的 ATP 结合和水解对于分子伴侣是必不可少的。然而,NBD-1 突变体显示出分子伴侣活性,但活性因突变的性质而异。我们的结果表明,与其他 HSP100 家族伴侣不同,ClpL 是一种新型伴侣,其活性不需要任何额外的二级伴侣。
更新日期:2020-09-15
中文翻译:

ClpL 单个域的意义:来自变形链球菌的新型伴侣。
ClpL 是 AAA+ 分子伴侣的 HSP100 家族成员,广泛存在于革兰氏阳性菌中,但出人意料地不存在于革兰氏阴性菌中。ClpL 参与各种细胞过程,包括应激耐受反应、长期存活、毒力和抗生素抗性。ClpL 的特征很差,其分子伴侣活性的分子机制尚不清楚。在这里,我们对来自变形链球菌的 ClpL 蛋白进行了生化表征,一种牙科病原体,以了解其生物学功能。ClpL 包含五个域:N 域、两个核苷酸结合域(NBD-1 和 NBD-2)、M 域和 C 域。NBD-1 和 NBD-2 分别包含不同的 Walker A 和 B 基序,用于 ATP 结合和水解。我们发现 ClpL 在溶液中主要以三聚体形式存在;然而,在与 ATP 结合后,它会迅速形成六聚体结构。为了研究结构-功能活性,我们构建了几个替换和缺失突变体。我们发现 Walker A 和 B 基序中的突变干扰了 ATP 水解和寡聚化。同样,N-、M-和C-结构域的缺失消除了ATPase 活性和寡聚化。因为我们之前发现 ClpL 充当伴侣,所以我们分析了伴侣活动。出奇,我们发现 NBD-2 突变体没有显示任何分子伴侣活性,表明 NBD-2 的 ATP 结合和水解对于分子伴侣是必不可少的。然而,NBD-1 突变体显示出分子伴侣活性,但活性因突变的性质而异。我们的结果表明,与其他 HSP100 家族伴侣不同,ClpL 是一种新型伴侣,其活性不需要任何额外的二级伴侣。











































 京公网安备 11010802027423号
京公网安备 11010802027423号