当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electric Field Mediated Selectivity Switching of Electrochemical CO2 Reduction from Formate to CO on Carbon Supported Sn
ACS Energy Letters ( IF 19.3 ) Pub Date : 2020-08-12 , DOI: 10.1021/acsenergylett.0c01387 Mi-Young Lee 1 , Stefan Ringe 2 , Hyungjun Kim 3 , Seoktae Kang 1 , Youngkook Kwon 4
ACS Energy Letters ( IF 19.3 ) Pub Date : 2020-08-12 , DOI: 10.1021/acsenergylett.0c01387 Mi-Young Lee 1 , Stefan Ringe 2 , Hyungjun Kim 3 , Seoktae Kang 1 , Youngkook Kwon 4
Affiliation
|
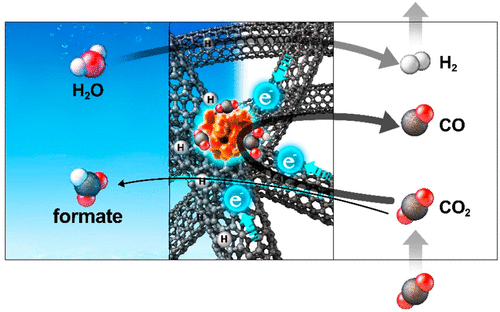
Decades of electrochemical CO2 reduction research have led to established rules about the product selectivity, i.e., bare tin yields formic acid as the main product. Here, we present Sn nanoparticles supported on carbon nanotubes (CNTs) in a hollow fiber (Sn-CHF), which produce CO with 10 times higher selectivity than formate. Density functional theory calculations reveal that a strong interfacial field induced by the carbon support enhances the rate-limiting CO2 adsorption and thus CO production on Sn nanoparticles, whereas the field-insensitive formate and hydrogen production routes were completely suppressed and occurred mainly from carbon sites. Modification of the interfacial electric field via exchange of the electrolyte-containing cation from Li+ to Cs+ induces an unprecedented 2 orders of magnitude change in the CO current while keeping the other products almost unchanged. This work demonstrates how electrochemical selectivity rules can be modulated by controlling the interfacial field, thus opening up new windows for electrocatalyst design.
中文翻译:

碳载锡上电场催化电化学还原CO 2从甲酸酯转化为CO的选择性转换
数十年来的电化学还原CO 2的研究已经形成了关于产物选择性的既定规则,即裸露的锡以甲酸为主要产物。在这里,我们介绍了中空纤维(Sn-CHF)中负载在碳纳米管(CNT)上的Sn纳米颗粒,该纳米颗粒产生的CO选择性是甲酸的10倍。密度泛函理论计算表明,碳载体诱导的强界面电场增强了限速CO 2吸附,从而提高了Sn纳米颗粒上的CO生成,而对电场不敏感的甲酸和氢的生成途径被完全抑制,并且主要发生在碳位点。通过将含电解质的阳离子从Li +交换为Cs来改变界面电场+导致CO电流发生前所未有的2个数量级的变化,而其他产品几乎保持不变。这项工作表明如何通过控制界面场来调节电化学选择性规则,从而为电催化剂设计打开新的窗口。
更新日期:2020-09-11
中文翻译:

碳载锡上电场催化电化学还原CO 2从甲酸酯转化为CO的选择性转换
数十年来的电化学还原CO 2的研究已经形成了关于产物选择性的既定规则,即裸露的锡以甲酸为主要产物。在这里,我们介绍了中空纤维(Sn-CHF)中负载在碳纳米管(CNT)上的Sn纳米颗粒,该纳米颗粒产生的CO选择性是甲酸的10倍。密度泛函理论计算表明,碳载体诱导的强界面电场增强了限速CO 2吸附,从而提高了Sn纳米颗粒上的CO生成,而对电场不敏感的甲酸和氢的生成途径被完全抑制,并且主要发生在碳位点。通过将含电解质的阳离子从Li +交换为Cs来改变界面电场+导致CO电流发生前所未有的2个数量级的变化,而其他产品几乎保持不变。这项工作表明如何通过控制界面场来调节电化学选择性规则,从而为电催化剂设计打开新的窗口。










































 京公网安备 11010802027423号
京公网安备 11010802027423号