当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Redesign of Substrate Selection in Glycopeptide Antibiotic Biosynthesis Enables Effective Formation of Alternate Peptide Backbones.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-08-14 , DOI: 10.1021/acschembio.0c00435 Milda Kaniusaite 1, 2 , Tiia Kittilä 3 , Robert J A Goode 1, 4 , Ralf B Schittenhelm 1, 4 , Max J Cryle 1, 2
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-08-14 , DOI: 10.1021/acschembio.0c00435 Milda Kaniusaite 1, 2 , Tiia Kittilä 3 , Robert J A Goode 1, 4 , Ralf B Schittenhelm 1, 4 , Max J Cryle 1, 2
Affiliation
|
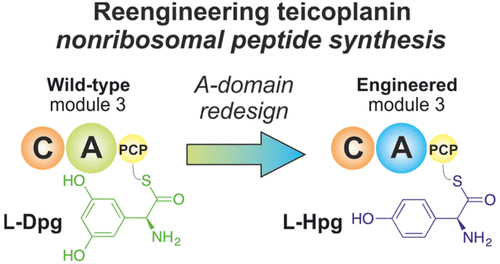
Nonribosomal peptide synthesis is capable of utilizing a wide range of amino acid residues due to the selectivity of adenylation (A)-domains. Changing the selectivity of A-domains could lead to new bioactive nonribosomal peptides, although remodeling efforts of A-domains are often unsuccessful. Here, we explored and successfully reengineered the specificity of the module 3 A-domain from glycopeptide antibiotic biosynthesis to change the incorporation of 3,5-dihydroxyphenylglycine into 4-hydroxyphenylglycine. These engineered A-domains remain selective in a functioning peptide assembly line even under substrate competition conditions and indicate a possible application of these for the future redesign of GPA biosynthesis.
中文翻译:

糖肽抗生素生物合成中底物选择的重新设计使替代肽骨架的有效形成成为可能。
由于腺苷酸化(A)域的选择性,非核糖体肽合成能够利用广泛的氨基酸残基。改变A结构域的选择性可能会导致新的具有生物活性的非核糖体肽,尽管A结构域的重塑工作通常是不成功的。在这里,我们探索并成功地重新设计了糖肽类抗生素生物合成模块3A结构域的特异性,以改变3,5-二羟基苯基甘氨酸向4-羟基苯基甘氨酸的掺入。即使在底物竞争条件下,这些工程化的A结构域在功能正常的肽装配线中仍具有选择性,并表明它们在将来重新设计GPA生物合成中的可能应用。
更新日期:2020-09-20
中文翻译:

糖肽抗生素生物合成中底物选择的重新设计使替代肽骨架的有效形成成为可能。
由于腺苷酸化(A)域的选择性,非核糖体肽合成能够利用广泛的氨基酸残基。改变A结构域的选择性可能会导致新的具有生物活性的非核糖体肽,尽管A结构域的重塑工作通常是不成功的。在这里,我们探索并成功地重新设计了糖肽类抗生素生物合成模块3A结构域的特异性,以改变3,5-二羟基苯基甘氨酸向4-羟基苯基甘氨酸的掺入。即使在底物竞争条件下,这些工程化的A结构域在功能正常的肽装配线中仍具有选择性,并表明它们在将来重新设计GPA生物合成中的可能应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号