当前位置:
X-MOL 学术
›
ACS Appl. Polym. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unconventional Conjugation via vinylMeSi(O−)2 Siloxane Bridges May Imbue Semiconducting Properties in [vinyl(Me)SiO(PhSiO1.5)8OSi(Me)vinyl-Ar] Double-Decker Copolymers
ACS Applied Polymer Materials ( IF 5 ) Pub Date : 2020-08-13 , DOI: 10.1021/acsapm.0c00591 J. Guan 1 , J. J. R. Arias 1, 2 , K. Tomobe 3 , R. Ansari 4 , M. de F. V. Marques 2 , A. Rebane 5, 6 , S. Mahbub 7 , J. C. Furgal 7 , N. Yodsin 8 , S. Jungsuttiwong 8 , D. Hashemi 1 , J. Kieffer 1 , R. M. Laine 1, 9
ACS Applied Polymer Materials ( IF 5 ) Pub Date : 2020-08-13 , DOI: 10.1021/acsapm.0c00591 J. Guan 1 , J. J. R. Arias 1, 2 , K. Tomobe 3 , R. Ansari 4 , M. de F. V. Marques 2 , A. Rebane 5, 6 , S. Mahbub 7 , J. C. Furgal 7 , N. Yodsin 8 , S. Jungsuttiwong 8 , D. Hashemi 1 , J. Kieffer 1 , R. M. Laine 1, 9
Affiliation
|
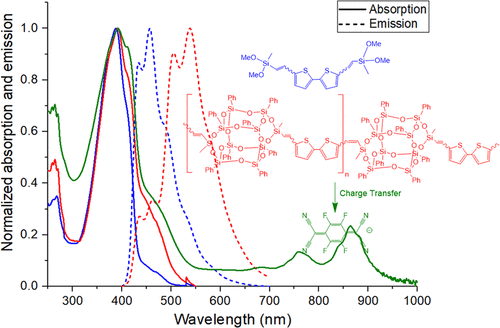
A number of groups have invested considerable time synthesizing double-decker silsesquioxane (DD SQ) copolymers; however, to our knowledge, no one has sought to explore through-chain electronic communication between DD SQs via “conjugated” co-monomers. We recently demonstrated that stilbene derivatives of simple DD cages exhibit properties commensurate with formation of cage centered lowest unoccupied molecular orbitals (LUMOs), equivalent to LUMOs found in complete/incomplete SQ cages, [RStilbeneSiO1.5]8,10,12, [RStilbeneSiO1.5]7[O1.5SiMe/nPr], [RStilbeneSiO1.5]7[O0.5SiMe3]3, [RStilbeneSiO1.5]8[O0.5-SiMe3]4, and [RStilbeneSiO1.5]8[OSiMe2]2. Such LUMOs support the existence of 3D excited-state conjugation in these cages. We describe here Heck catalyzed copolymerization of vinyl(Me)SiO(PhSiO1.5)8OSi(Me)vinyl (vinylDDvinyl) with X–Ar–X, where X = Br or I and X–Ar–X = 1,4-dihalobenzene, 4,4′-dibromo-1,1′-biphenyl, 4,4″-dibromo-p-terphenyl, 4,4′-dibromo-trans-stilbene, 2,5-dibromothiophene, 5,5′-dibromo-2,2′-bithiophene, 2,5-dibromothieno[3,2-b]thiophene, and 2,7-dibromo-9,9-dimethylfluorene. Coincidentally model analogs were synthesized from vinylMeSi(OMe)2. All compounds were characterized in detail by gel permeation chromatography (GPC), matrix-assisted laser desorption/ionization-time-of-flight, thermogravimetric analysis, nuclear magnetic resonance, Fourier transfer infrared spectroscopy, ultraviolet–visible spectroscopy, photoluminescence spectrometry, and two-photon absorption (2PA) spectroscopy. Modeling of HOMO–LUMO energy levels of related compounds with R = Me rather than Ph was also explored. In the current systems, we again see apparent conjugation in excited states, as previously observed, as indicated by 50–120 nm red shifts in emission from the corresponding model silane compounds. These results suggest unexpected semiconducting behavior via vinylMeSi(O−)2 (siloxane) bridges between DD cages in polymers. The thiophene, bithiophene, and thienothiophene copolymers display integer charge transfer behavior on doping with 10 mol % F4TCNQ supporting excited-state conjugation; suggesting potential as p-type, doped organic/inorganic semiconductors.
中文翻译:

通过vinylMeSi(O-)2硅氧烷桥进行的非常规共轭可能影响[vinyl(Me)SiO(PhSiO 1.5)8 OSi(Me)vinyl-Ar]双层共聚物中的半导体性能
许多小组投入了大量时间来合成双层倍半硅氧烷(DD SQ)共聚物。然而,据我们所知,没有人试图通过“共轭”共聚单体探索DD SQ之间的全链电子通信。我们最近证明,简单DD笼的二苯乙烯衍生物表现出与以笼中心为中心的最低未占据分子轨道(LUMOs)形成相称的特性,相当于在完全/不完全SQ笼中发现的LUMO,[RStilbeneSiO 1.5 ] 8,10,12,[RStilbeneSiO 1.5 ] 7 [O 1.5 SiMe / nPr],[RStilbeneSiO 1.5 ] 7 [O 0.5 SiMe 3 ] 3,[RStilbeneSiO 1.5 ] 8 [O 0.5- SiMe 3 ] 4和[RStilbeneSiO 1.5 ] 8 [OSiMe 2 ] 2。这种LUMO支持这些笼子中3D激发态共轭的存在。我们在这里描述了Heck催化的乙烯基(Me)SiO(PhSiO 1.5)8 OSi(Me)乙烯基(乙烯基DD乙烯基)与X–Ar–X的共聚反应,其中X = Br或I,X–Ar–X = 1,4-二卤苯,4,4'-二溴-1,1'-联苯,4,4 ''-二溴-对-三联苯,4,4'-二溴-反式-sti,2,5-二溴噻吩,5,5'-二溴- 2,2'-联噻吩,2,5-二溴噻吩[3,2- b]噻吩和2,7-二溴-9,9-二甲基芴。巧合的是,由vinylMeSi(OMe)2合成模型类似物。所有化合物均通过凝胶渗透色谱(GPC),基质辅助激光解吸/电离飞行时间,热重分析,核磁共振,傅立叶转移红外光谱,紫外可见光谱,光致发光光谱和两种方法进行了详细表征-光子吸收(2PA)光谱。还探索了用R = Me而不是Ph对相关化合物的HOMO–LUMO能级进行建模的方法。在当前系统中,我们再次看到在激发态下出现了明显的共轭,如先前观察到的,这是由相应的模型硅烷化合物发射的50-120 nm红移所表明的。这些结果表明,通过vinylMeSi(O-)2发生了意外的半导体行为(硅氧烷)在DD笼之间的桥接。噻吩,联噻吩和噻吩并噻吩共聚物在掺杂10 mol%F 4 TCNQ时,表现出整数的电荷转移行为,从而支持激发态共轭。暗示有可能成为p型掺杂有机/无机半导体。
更新日期:2020-09-11
中文翻译:

通过vinylMeSi(O-)2硅氧烷桥进行的非常规共轭可能影响[vinyl(Me)SiO(PhSiO 1.5)8 OSi(Me)vinyl-Ar]双层共聚物中的半导体性能
许多小组投入了大量时间来合成双层倍半硅氧烷(DD SQ)共聚物。然而,据我们所知,没有人试图通过“共轭”共聚单体探索DD SQ之间的全链电子通信。我们最近证明,简单DD笼的二苯乙烯衍生物表现出与以笼中心为中心的最低未占据分子轨道(LUMOs)形成相称的特性,相当于在完全/不完全SQ笼中发现的LUMO,[RStilbeneSiO 1.5 ] 8,10,12,[RStilbeneSiO 1.5 ] 7 [O 1.5 SiMe / nPr],[RStilbeneSiO 1.5 ] 7 [O 0.5 SiMe 3 ] 3,[RStilbeneSiO 1.5 ] 8 [O 0.5- SiMe 3 ] 4和[RStilbeneSiO 1.5 ] 8 [OSiMe 2 ] 2。这种LUMO支持这些笼子中3D激发态共轭的存在。我们在这里描述了Heck催化的乙烯基(Me)SiO(PhSiO 1.5)8 OSi(Me)乙烯基(乙烯基DD乙烯基)与X–Ar–X的共聚反应,其中X = Br或I,X–Ar–X = 1,4-二卤苯,4,4'-二溴-1,1'-联苯,4,4 ''-二溴-对-三联苯,4,4'-二溴-反式-sti,2,5-二溴噻吩,5,5'-二溴- 2,2'-联噻吩,2,5-二溴噻吩[3,2- b]噻吩和2,7-二溴-9,9-二甲基芴。巧合的是,由vinylMeSi(OMe)2合成模型类似物。所有化合物均通过凝胶渗透色谱(GPC),基质辅助激光解吸/电离飞行时间,热重分析,核磁共振,傅立叶转移红外光谱,紫外可见光谱,光致发光光谱和两种方法进行了详细表征-光子吸收(2PA)光谱。还探索了用R = Me而不是Ph对相关化合物的HOMO–LUMO能级进行建模的方法。在当前系统中,我们再次看到在激发态下出现了明显的共轭,如先前观察到的,这是由相应的模型硅烷化合物发射的50-120 nm红移所表明的。这些结果表明,通过vinylMeSi(O-)2发生了意外的半导体行为(硅氧烷)在DD笼之间的桥接。噻吩,联噻吩和噻吩并噻吩共聚物在掺杂10 mol%F 4 TCNQ时,表现出整数的电荷转移行为,从而支持激发态共轭。暗示有可能成为p型掺杂有机/无机半导体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号