当前位置:
X-MOL 学术
›
Pept. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering and characterization of a pH‐sensitive homodimeric antiparallel coiled coil
Peptide Science ( IF 1.5 ) Pub Date : 2020-08-14 , DOI: 10.1002/pep2.24180 Radhika P. Nagarkar 1 , Galit Fichman 2 , Joel P. Schneider 2
Peptide Science ( IF 1.5 ) Pub Date : 2020-08-14 , DOI: 10.1002/pep2.24180 Radhika P. Nagarkar 1 , Galit Fichman 2 , Joel P. Schneider 2
Affiliation
|
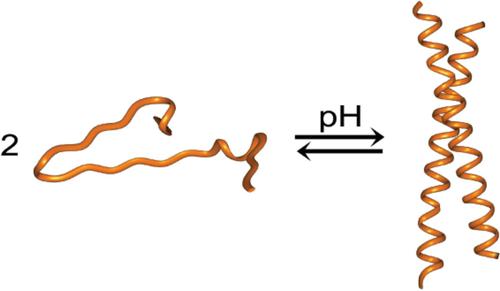
Structure‐based engineering starting from the antiparallel coiled coil domain of the Bcr‐Abl oncoprotein (BCR30‐65) afforded a new homodimeric antiparallel coiled coil whose folding is responsive to solution pH. The BCR30‐65 homodimer contains two glutamic acids (Glu52) buried in its hydrophobic core which one would expect to impart pH responsive folding behavior. Surprisingly, BCR30‐65 is resistant to thermal denaturation over a wide pH range. This behavior arises from stabilizing salt bridges formed between solvent‐exposed Arg55 and Glu52 that passivates the glutamates’ negative charge within the protein’s core. Replacing Arg55 with alanine imparts pH‐responsive folding behavior, but also results in the loss of directional specificity between the coiled coil’s helices. We find that an Ile42Glu mutation preserves the salt bridges and imparts the desired pH responsive folding behavior. The Ile42Glu protein forms an antiparallel homodimeric coiled coil under acidic conditions but unfolds under basic conditions and is only marginally less thermally stable compared to BCR30‐65.
中文翻译:

pH敏感的同二聚体反平行卷曲线圈的工程设计和表征
从Bcr-Abl癌蛋白(BCR 30-65)的反平行卷曲螺旋结构域开始的基于结构的工程设计提供了一种新的同二聚体反平行卷曲螺旋结构,其折叠对溶液pH响应。BCR 30-65同型二聚体在其疏水性核中掩埋了两种谷氨酸(Glu52),其中一种有望赋予pH响应折叠特性。令人惊讶的是,BCR 30-65在很宽的pH范围内具有抗热变性的能力。这种现象是由于稳定的,在溶剂暴露的Arg55和Glu52之间形成的盐桥而钝化了蛋白质核心中谷氨酸盐的负电荷。用丙氨酸代替Arg55可以赋予pH响应折叠行为,但同时也会导致卷曲螺旋之间的方向特异性丧失。我们发现,Ile42Glu突变保留了盐桥并赋予了所需的pH响应折叠行为。Ile42Glu蛋白在酸性条件下会形成反平行的同二聚体卷曲螺旋,但在碱性条件下会展开,并且与BCR 30-65相比,其热稳定性仅稍差一些。
更新日期:2020-09-26
中文翻译:

pH敏感的同二聚体反平行卷曲线圈的工程设计和表征
从Bcr-Abl癌蛋白(BCR 30-65)的反平行卷曲螺旋结构域开始的基于结构的工程设计提供了一种新的同二聚体反平行卷曲螺旋结构,其折叠对溶液pH响应。BCR 30-65同型二聚体在其疏水性核中掩埋了两种谷氨酸(Glu52),其中一种有望赋予pH响应折叠特性。令人惊讶的是,BCR 30-65在很宽的pH范围内具有抗热变性的能力。这种现象是由于稳定的,在溶剂暴露的Arg55和Glu52之间形成的盐桥而钝化了蛋白质核心中谷氨酸盐的负电荷。用丙氨酸代替Arg55可以赋予pH响应折叠行为,但同时也会导致卷曲螺旋之间的方向特异性丧失。我们发现,Ile42Glu突变保留了盐桥并赋予了所需的pH响应折叠行为。Ile42Glu蛋白在酸性条件下会形成反平行的同二聚体卷曲螺旋,但在碱性条件下会展开,并且与BCR 30-65相比,其热稳定性仅稍差一些。











































 京公网安备 11010802027423号
京公网安备 11010802027423号