当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of novel thiourea‐stilbene‐triazine conjugates as persuasive lymphoid tyrosine phosphatase inhibitors
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-08-14 , DOI: 10.1002/jhet.4060 Iram Batool 1 , Farukh Jabeen 1 , Nadeem Ahmed Vellore 2 , Ghulam Shabir 1 , Aamer Saeed 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-08-14 , DOI: 10.1002/jhet.4060 Iram Batool 1 , Farukh Jabeen 1 , Nadeem Ahmed Vellore 2 , Ghulam Shabir 1 , Aamer Saeed 1
Affiliation
|
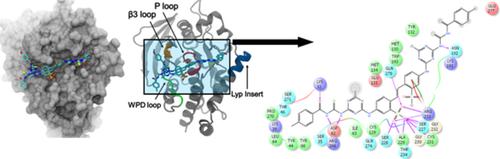
A library of novel thiourea‐based symmetrical stilbene‐triazines (5a‐i) was synthesized in an effort to develop new protein tyrosine phosphatase LYP inhibitors. The versatile nature of 2,4,6‐trichloro‐1,3,5‐triazine allows considerable scope for derivatization and hence exploration of structure activity relationships. A convenient and versatile three‐step synthetic approach involved the successive replacement of the two chloro groups of 2,4,6‐trichloro‐1,3,5‐triazine by a variety of substituents for structural modification. The newly synthesized derivatives were subjected to tyrosine phosphatase LYP inhibition studies. The results for the in vitro bioassays were promising with the identification of compound 5k and 5l having a 4‐methyl and 4‐methoxy substituent on phenyl ring, as the lead and selective candidate for LYP inhibition with an IC50 value of 2.1 ± 0.05 μM and 28 ± 3.3 μM, respectively. Moreover, docking studies were carried out to determine the possible interaction sites of thiourea‐based stilbene‐triazine compounds with Lymphoid Tyrosine Phosphatase. Results of docking computations further ascertained the inhibitory potential of compound 5k and 5l. The results indicated that the compound 5k may serve as a structural model for the design of most potent LYP inhibitors.
中文翻译:

鉴定新型硫脲-二苯乙烯-三嗪共轭物作为有说服力的淋巴样酪氨酸磷酸酶抑制剂
为了开发新的蛋白质酪氨酸磷酸酶LYP抑制剂,合成了一个基于硫脲的对称二苯乙烯-三嗪新化合物(5a-i)库。2,4,6-三氯-1,3,5-三嗪的通用性质为衍生化提供了广阔的空间,因此也探索了结构活性关系。一种方便且通用的三步合成方法涉及用各种取代基连续取代2,4,6-三氯-1,3,5-三嗪的两个氯基,以进行结构修饰。对新合成的衍生物进行酪氨酸磷酸酶LYP抑制研究。化合物5k和5l的鉴定为体外生物测定的结果带来了希望在苯环上具有4-甲基和4-甲氧基取代基,作为LYP抑制的铅和选择性候选物,IC 50值分别为2.1±0.05μM和28±3.3μM。此外,进行了对接研究,以确定基于硫脲的二苯乙烯-三嗪化合物与淋巴酪氨酸磷酸酶的可能相互作用位点。对接计算的结果进一步确定了化合物5k和5l的抑制潜力。结果表明,化合物5k可用作设计最有效的LYP抑制剂的结构模型。
更新日期:2020-09-08
中文翻译:

鉴定新型硫脲-二苯乙烯-三嗪共轭物作为有说服力的淋巴样酪氨酸磷酸酶抑制剂
为了开发新的蛋白质酪氨酸磷酸酶LYP抑制剂,合成了一个基于硫脲的对称二苯乙烯-三嗪新化合物(5a-i)库。2,4,6-三氯-1,3,5-三嗪的通用性质为衍生化提供了广阔的空间,因此也探索了结构活性关系。一种方便且通用的三步合成方法涉及用各种取代基连续取代2,4,6-三氯-1,3,5-三嗪的两个氯基,以进行结构修饰。对新合成的衍生物进行酪氨酸磷酸酶LYP抑制研究。化合物5k和5l的鉴定为体外生物测定的结果带来了希望在苯环上具有4-甲基和4-甲氧基取代基,作为LYP抑制的铅和选择性候选物,IC 50值分别为2.1±0.05μM和28±3.3μM。此外,进行了对接研究,以确定基于硫脲的二苯乙烯-三嗪化合物与淋巴酪氨酸磷酸酶的可能相互作用位点。对接计算的结果进一步确定了化合物5k和5l的抑制潜力。结果表明,化合物5k可用作设计最有效的LYP抑制剂的结构模型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号