当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
HFO-1234yf as a CF3-building block: Synthesis and Chemistry of CF3-ynones
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2020-09-10 , DOI: 10.1002/ejoc.202001071 Ben J. Murray 1 , Thomas G. F. Marsh 1 , Dmitri S. Yufit 1 , Mark A. Fox 1 , Antal Harsanyi 1 , Lee T. Boulton 2 , Graham Sandford 3
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2020-09-10 , DOI: 10.1002/ejoc.202001071 Ben J. Murray 1 , Thomas G. F. Marsh 1 , Dmitri S. Yufit 1 , Mark A. Fox 1 , Antal Harsanyi 1 , Lee T. Boulton 2 , Graham Sandford 3
Affiliation
|
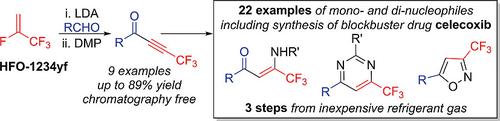
Reaction of low cost, readily available 4th generation refrigerant gas 2,3,3,3‐tetrafluoropropene (HFO‐1234yf) with lithium diisopropylamide (LDA) leads to formation of lithium 3,3,3‐trifluoropropynide, addition of which to a range of aldehydes formed CF3‐alkynyl alcohol derivatives on multigram scale, which were oxidised using Dess‐Martin periodinane (DMP) to give substituted CF3‐ynones with minimal purification required. Michael‐type additions of alcohol and amine nucleophiles to CF3‐ynones are rapid and selective, affording a range of CF3‐enone ethers and enaminones in excellent yields with high stereoselectivity for the Z‐isomer. By analogous reactions with difunctional nucleophiles, a wide range of CF3‐substituted pharmaceutically relevant heterocyclic structures can be accessed, exemplified in the simple synthesis of the anti‐arthritis drug celecoxib from HFO‐1234yf in just three steps.
中文翻译:

HFO-1234yf 作为 CF3 构建块:CF3-炔酮的合成和化学
低成本、易得的第 4 代制冷剂气体 2,3,3,3-四氟丙烯 (HFO-1234yf) 与二异丙基氨基锂 (LDA) 的反应导致形成 3,3,3-三氟丙炔化锂,添加到一定范围内醛形成数克规模的 CF3-炔醇衍生物,使用戴斯-马丁高碘烷 (DMP) 将其氧化以得到取代的 CF3-炔酮,所需的纯化最少。醇和胺亲核试剂向 CF3-炔酮的迈克尔型加成是快速和选择性的,以优异的收率提供一系列 CF3-烯酮醚和烯胺酮,对 Z-异构体具有高立体选择性。通过与双功能亲核试剂的类似反应,可以获得广泛的 CF3 取代的药学相关杂环结构,
更新日期:2020-09-10
中文翻译:

HFO-1234yf 作为 CF3 构建块:CF3-炔酮的合成和化学
低成本、易得的第 4 代制冷剂气体 2,3,3,3-四氟丙烯 (HFO-1234yf) 与二异丙基氨基锂 (LDA) 的反应导致形成 3,3,3-三氟丙炔化锂,添加到一定范围内醛形成数克规模的 CF3-炔醇衍生物,使用戴斯-马丁高碘烷 (DMP) 将其氧化以得到取代的 CF3-炔酮,所需的纯化最少。醇和胺亲核试剂向 CF3-炔酮的迈克尔型加成是快速和选择性的,以优异的收率提供一系列 CF3-烯酮醚和烯胺酮,对 Z-异构体具有高立体选择性。通过与双功能亲核试剂的类似反应,可以获得广泛的 CF3 取代的药学相关杂环结构,











































 京公网安备 11010802027423号
京公网安备 11010802027423号