当前位置:
X-MOL 学术
›
Chemistryopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Curcumin Conjugates of Non-steroidal Anti-Inflammatory Drugs: Synthesis, Structures, Anti-proliferative Assays, Computational Docking, and Inflammatory Response.
ChemistryOpen ( IF 2.5 ) Pub Date : 2020-08-13 , DOI: 10.1002/open.202000173 Kenneth K Laali 1 , Angela T Zwarycz 1 , Nicholas Beck 1 , Gabriela L Borosky 2 , Manabu Nukaya 3 , Gregory D Kennedy 3
ChemistryOpen ( IF 2.5 ) Pub Date : 2020-08-13 , DOI: 10.1002/open.202000173 Kenneth K Laali 1 , Angela T Zwarycz 1 , Nicholas Beck 1 , Gabriela L Borosky 2 , Manabu Nukaya 3 , Gregory D Kennedy 3
Affiliation
|
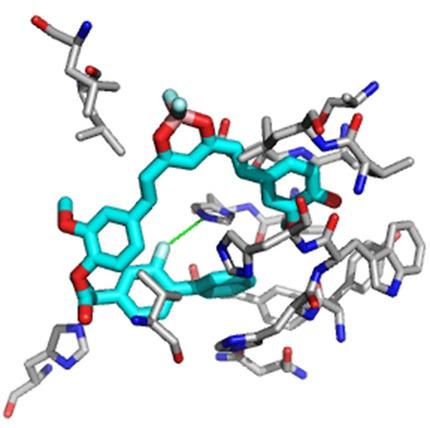
In an effort to combine the anti‐proliferative effect of CUR‐BF2 and CUR compounds with anti‐inflammatory benefits of non‐steroidal anti‐inflammatory drugs (NSAIDs), a library of the bis‐ and mono‐NSAID/CUR‐BF2 and NSAID/CUR conjugates were synthesized by coupling flufenamic acid, flurbiprofen, naproxen, indomethacin, and ibuprofen to diversely substituted hydroxy‐benzaldehydes via an ester linkage, and by subsequent reaction with acetylacetone‐BF2 to form the bis‐ and the mono‐NSAID/CUR‐BF2 adducts. Since conversion to NSAID/CUR by the previously developed decomplexation protocol showed limited success, a set of NSAID/CUR conjugates were independently prepared by directly coupling the NSAIDs with parent curcumin. The bis‐NSAID/CUR‐BF2 and bis‐NSAID‐CUR hybrids exhibited low cytotoxicity in NCI‐60 assay, and in independent cell viability assay on colorectal cancer (CRC) cells (HCT116, HT29, DLD‐1, RKO, SW837, CaCo2) and in normal CR cells (CCD841CoN). By contrast, the mono‐naproxin and mono‐flurbiprofen CUR‐BF2 adducts exhibited remarkable anti‐proliferative and apoptopic activity in NCI‐60 assay most notably against HCT‐116 (colon), OVCAR‐3 (ovarian), and ACHN (renal) cells. Computational molecular docking calculations showed favorable binding energies to HER2, VEGFR2, BRAF, and Bcl‐2 as well as to COX‐1 and COX‐2, which in several cases exceeded known inhibitors. The main interactions between the ligands and the proteins were hydrophobic, although several hydrogen bonds were also observed. A sub‐set of six compounds that had exhibited little or no cytotoxicity were tested for their anti‐inflammatory response with THP‐1 human macrophages in comparison to parent NSAIDs or parent curcumin.
中文翻译:

非甾体抗炎药的姜黄素缀合物:合成、结构、抗增殖测定、计算对接和炎症反应。
为了将 CUR-BF 2和 CUR 化合物的抗增殖作用与非甾体抗炎药 (NSAID) 的抗炎作用相结合,建立了双- 和单-NSAID/CUR-BF 2库和 NSAID/CUR 缀合物的合成方法是通过酯键将氟芬那酸、氟比洛芬、萘普生、吲哚美辛和布洛芬与不同取代的羟基苯甲醛偶联,然后与乙酰丙酮-BF 2反应形成双-和单-NSAID /CUR-BF 2加合物。由于通过先前开发的解络方案转化为 NSAID/CUR 的成功有限,因此通过直接将 NSAID 与母体姜黄素偶联来独立制备一组 NSAID/CUR 缀合物。双-NSAID/CUR-BF 2和双-NSAID-CUR 杂合体在 NCI-60 测定中以及在结直肠癌 (CRC) 细胞(HCT116、HT29、DLD-1、RKO、SW837)的独立细胞活力测定中表现出低细胞毒性、CaCo2) 和正常 CR 细胞 (CCD841CoN)。相比之下,单萘普生和单氟比洛芬 CUR-BF 2加合物在 NCI-60 测定中表现出显着的抗增殖和细胞凋亡活性,最显着的是针对 HCT-116(结肠)、OVCAR-3(卵巢)和 ACHN(肾) )细胞。计算分子对接计算显示与 HER2、VEGFR2、BRAF 和 Bcl-2 以及 COX-1 和 COX-2 具有良好的结合能,在一些情况下超过了已知的抑制剂。配体和蛋白质之间的主要相互作用是疏水性的,尽管也观察到了几个氢键。 与母体 NSAID 或母体姜黄素相比,测试了六种几乎没有或没有细胞毒性的化合物的子集对 THP-1 人巨噬细胞的抗炎反应。
更新日期:2020-08-13
中文翻译:

非甾体抗炎药的姜黄素缀合物:合成、结构、抗增殖测定、计算对接和炎症反应。
为了将 CUR-BF 2和 CUR 化合物的抗增殖作用与非甾体抗炎药 (NSAID) 的抗炎作用相结合,建立了双- 和单-NSAID/CUR-BF 2库和 NSAID/CUR 缀合物的合成方法是通过酯键将氟芬那酸、氟比洛芬、萘普生、吲哚美辛和布洛芬与不同取代的羟基苯甲醛偶联,然后与乙酰丙酮-BF 2反应形成双-和单-NSAID /CUR-BF 2加合物。由于通过先前开发的解络方案转化为 NSAID/CUR 的成功有限,因此通过直接将 NSAID 与母体姜黄素偶联来独立制备一组 NSAID/CUR 缀合物。双-NSAID/CUR-BF 2和双-NSAID-CUR 杂合体在 NCI-60 测定中以及在结直肠癌 (CRC) 细胞(HCT116、HT29、DLD-1、RKO、SW837)的独立细胞活力测定中表现出低细胞毒性、CaCo2) 和正常 CR 细胞 (CCD841CoN)。相比之下,单萘普生和单氟比洛芬 CUR-BF 2加合物在 NCI-60 测定中表现出显着的抗增殖和细胞凋亡活性,最显着的是针对 HCT-116(结肠)、OVCAR-3(卵巢)和 ACHN(肾) )细胞。计算分子对接计算显示与 HER2、VEGFR2、BRAF 和 Bcl-2 以及 COX-1 和 COX-2 具有良好的结合能,在一些情况下超过了已知的抑制剂。配体和蛋白质之间的主要相互作用是疏水性的,尽管也观察到了几个氢键。 与母体 NSAID 或母体姜黄素相比,测试了六种几乎没有或没有细胞毒性的化合物的子集对 THP-1 人巨噬细胞的抗炎反应。










































 京公网安备 11010802027423号
京公网安备 11010802027423号