The Journal of Supercritical Fluids ( IF 3.9 ) Pub Date : 2020-08-14 , DOI: 10.1016/j.supflu.2020.105023 Andrew Mansfield
|
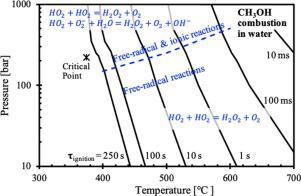
A revised detailed chemical kinetic mechanism for the combustion of methanol in sub and supercritical water was developed, which is accurate across a wide range of thermodynamic states relevant to hydrothermal combustion. Development was accomplished by revising an existing mechanism for air dilute combustion of methanol at elevated pressures, to include a real gas model and reaction rate modifications relevant to hydrothermal combustion kinetics. Subsequent analysis using the revised mechanism revealed that trends in auto-ignition delay time and dominant reaction pathways are relatively invariant across sub and supercritical states, even near the critical point. While reaction pathways were consistent with previous findings, the addition of a formic acid pathway from formaldehyde to CO2 was found to be important. Analysis results also support a hypothesis that formation of the superoxide ion at higher pressure lower temperature conditions enhances the rate of self-termination of HO2, through the activation of an alternative pathway, HO2 + O2− + H2O = H2O2 + O2 + OH−.
中文翻译:

修正的亚临界和超临界水中甲醇燃烧的化学动力学机理
开发了修订的详细化学动力学机制,用于在亚临界水和超临界水中燃烧甲醇,该机制在涉及水热燃烧的各种热力学状态下均十分精确。通过修改现有的在高压下进行甲醇空气稀薄燃烧的机制来完成开发,以包括真实的气体模型和与水热燃烧动力学相关的反应速率修正。随后使用修订后的机制进行的分析表明,自燃延迟时间和主要反应途径的趋势在亚临界和超临界状态甚至在临界点附近相对不变。虽然反应途径与以前的发现一致,但增加了从甲醛到CO 2的甲酸途径被发现很重要。分析结果也支持一个假设,即形成在较高的压力下的温度条件下的超氧离子的增强HO的自终止的速率2,通过替代途径的活化,HO 2 + O 2 - + H 2 O = H ^ 2 Ò 2 + O 2 + OH - 。



























 京公网安备 11010802027423号
京公网安备 11010802027423号