Structure ( IF 4.4 ) Pub Date : 2020-08-13 , DOI: 10.1016/j.str.2020.07.013 Rojan Shrestha 1 , Sarah C Garrett-Thomson 2 , Weifeng Liu 2 , Steven C Almo 2 , Andras Fiser 1
|
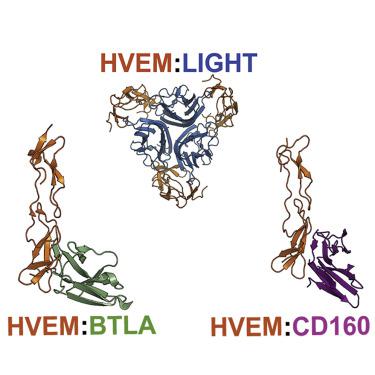
Herpes virus entry mediator (HVEM) regulates positive and negative signals for T cell activation through co-signaling pathways. Dysfunction of the HVEM co-signaling network is associated with multiple pathologies related to autoimmunity, infectious disease, and cancer, making the associated molecules biologically and therapeutically attractive targets. HVEM interacts with three ligands from two different superfamilies using two different binding interfaces. The engagement with ligands CD160 and B- and T-lymphocyte attenuator (BTLA), members of immunoglobulin superfamily, is associated with inhibitory signals, whereas inflammatory responses are regulated through the interaction with LIGHT from the TNF superfamily. We computationally redesigned the HVEM recognition interfaces using a residue-specific pharmacophore approach, ProtLID, to achieve switchable-binding specificity. In subsequent cell-based binding assays the new interfaces, designed with only single or double mutations, exhibited selective binding to only one or two out of the three cognate ligands.
中文翻译:

重新设计 HVEM 接口以选择性结合 LIGHT、BTLA 和 CD160。
疱疹病毒进入介质 (HVEM) 通过共信号通路调节 T 细胞活化的正负信号。HVEM 共信号网络的功能障碍与自身免疫、传染病和癌症相关的多种病理相关,使相关分子在生物学和治疗上具有吸引力。HVEM 使用两个不同的结合界面与来自两个不同超家族的三个配体相互作用。与配体 CD160 和 B 和 T 淋巴细胞衰减器 (BTLA)(免疫球蛋白超家族的成员)的结合与抑制信号有关,而炎症反应则通过与来自 TNF 超家族的 LIGHT 的相互作用进行调节。我们使用残基特异性药效团方法 ProtLID 计算重新设计了 HVEM 识别界面,以实现可切换的结合特异性。在随后的基于细胞的结合测定中,仅设计有单突变或双突变的新界面显示出与三种同源配体中仅一种或两种的选择性结合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号