Journal of Structural Biology ( IF 3.0 ) Pub Date : 2020-08-14 , DOI: 10.1016/j.jsb.2020.107605 Meng-Hsuan Lin , Po-Chih Kuo , Yi-Chih Chiu , Yu-Yung Chang , Sheng-Chia Chen , Chun-Hua Hsu
|
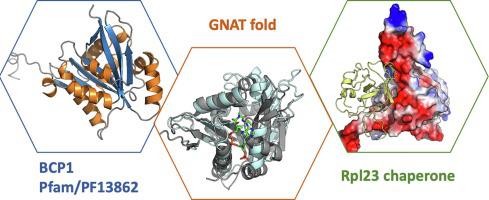
BCP1 is a protein enriched in the nucleus that is required for Mss4 nuclear export and identified as the chaperone of ribosomal protein Rpl23 in Saccharomyces cerevisiae. According to sequence homology, BCP1 is related to the mammalian BRCA2-interacting protein BCCIP and belongs to the BCIP protein family (PF13862) in the Pfam database. However, the BCIP family has no discernible similarity to proteins with known structure. Here, we report the crystal structure of BCP1, presenting an α/β fold in which the central antiparallel β-sheet is flanked by helices. Protein structural classification revealed that BCP1 has similarity to the GNAT superfamily but no conserved substrate-binding residues. Further modeling and protein–protein docking work provide a plausible model to explain the interaction between BCP1 and Rpl23. Our structural analysis presents the first structure of BCIP family and provides a foundation for understanding the molecular basis of BCP1 as a chaperone of Rpl23 for ribosome biosynthesis.
中文翻译:

来自酿酒酵母的蛋白质转运分子伴侣 BCP1 的晶体结构。
BCP1 是一种富含细胞核的蛋白质,它是 Mss4 核输出所必需的,被鉴定为酿酒酵母中核糖体蛋白 Rpl23 的分子伴侣. 根据序列同源性,BCP1与哺乳动物BRCA2相互作用蛋白BCCIP相关,属于Pfam数据库中的BCIP蛋白家族(PF13862)。然而,BCIP 家族与已知结构的蛋白质没有明显的相似性。在这里,我们报告了 BCP1 的晶体结构,呈现出 α/β 折叠,其中中央反平行的 β 折叠侧翼是螺旋。蛋白质结构分类显示 BCP1 与 GNAT 超家族具有相似性,但没有保守的底物结合残基。进一步的建模和蛋白质-蛋白质对接工作提供了一个合理的模型来解释 BCP1 和 Rpl23 之间的相互作用。我们的结构分析展示了 BCIP 家族的第一个结构,并为理解 BCP1 作为 Rpl23 分子伴侣进行核糖体生物合成的分子基础提供了基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号