Journal of Structural Biology ( IF 3 ) Pub Date : 2020-08-14 , DOI: 10.1016/j.jsb.2020.107604 Rafayel A Azizyan 1 , Weiqiang Wang 2 , Alexey Anikeenko 3 , Zinaida Radkova 3 , Anastasia Bakulina 3 , Adriana Garro 4 , Landry Charlier 5 , Christian Dumas 6 , Salvador Ventura 2 , Andrey V Kajava 1
|
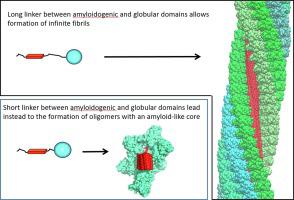
Insoluble amyloid fibrils formed by self-assembly of amyloidogenic regions of proteins have a cross-β-structure. In this work, by using targeted molecular dynamics and rigid body simulation, we demonstrate that if a protein consists of an amyloidogenic region and a globular domain(s) and if the linker between them is short enough, such molecules cannot assemble into amyloid fibrils, instead, they form oligomers with a defined and limited number of β-strands in the cross-β core. We show that this blockage of the amyloid growth is due to the steric repulsion of the globular structures linked to amyloidogenic regions. Furthermore, we establish a relationship between the linker length and the number of monomers in such nanoparticles. We hypothesise that such oligomerisation can be a yet unrecognised way to form natural protein complexes involved in biological processes. Our results can also be used in protein engineering for designing soluble nanoparticles carrying different functional domains.
中文翻译:

淀粉样蛋白原性作为形成功能性低聚物的驱动力。
由蛋白质的淀粉样蛋白区域自组装形成的不溶性淀粉样原纤维具有交叉 β 结构。在这项工作中,通过使用靶向分子动力学和刚体模拟,我们证明了如果蛋白质由淀粉样蛋白生成区域和球状结构域组成,并且它们之间的接头足够短,则此类分子无法组装成淀粉样蛋白原纤维,相反,它们在交叉 β 核心中形成具有确定且有限数量的 β 链的低聚物。我们表明这种淀粉样蛋白生长的阻塞是由于与淀粉样蛋白生成区域相关的球状结构的空间排斥。此外,我们建立了接头长度与此类纳米颗粒中单体数量之间的关系。我们假设这种寡聚化可能是一种尚未被认识的形成参与生物过程的天然蛋白质复合物的方式。我们的结果也可用于蛋白质工程,以设计携带不同功能域的可溶性纳米颗粒。



























 京公网安备 11010802027423号
京公网安备 11010802027423号