Acta Pharmaceutica Sinica B ( IF 14.7 ) Pub Date : 2020-08-13 , DOI: 10.1016/j.apsb.2020.07.025 Biyuan Wu , Jintao Fu , Yixian Zhou , Sulan Luo , Yiting Zhao , Guilan Quan , Xin Pan , Chuanbin Wu
|
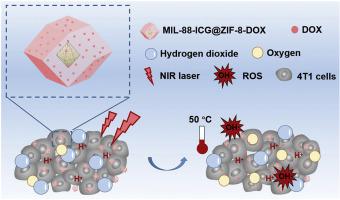
Malignant tumor has become an urgent threat to global public healthcare. Because of the heterogeneity of tumor, single therapy presents great limitations while synergistic therapy is arousing much attention, which shows desperate need of intelligent carrier for co-delivery. A core‒shell dual metal–organic frameworks (MOFs) system was delicately designed in this study, which not only possessed the unique properties of both materials, but also provided two individual specific functional zones for co-drug delivery. Photosensitizer indocyanine green (ICG) and chemotherapeutic agent doxorubicin (DOX) were stepwisely encapsulated into the nanopores of MIL-88 core and ZIF-8 shell to construct a synergistic photothermal/photodynamic/chemotherapy nanoplatform. Except for efficient drug delivery, the MIL-88 could be functioned as a nanomotor to convert the excessive hydrogen peroxide at tumor microenvironment into adequate oxygen for photodynamic therapy. The DOX release from MIL-88-ICG@ZIF-8-DOX nanoparticles was triggered at tumor acidic microenvironment and further accelerated by near-infrared (NIR) light irradiation. The in vivo antitumor study showed superior synergistic antitumor effect by concentrating the nanoparticles into dissolving microneedles as compared to intravenous and intratumoral injection of nanoparticles, with a significantly higher inhibition rate. It is anticipated that the multi-model synergistic system based on dual-MOFs was promising for further biomedical application.
中文翻译:

量身定制的核-壳双金属-有机骨架,可作为多功能纳米马达进行有效的协同抗肿瘤治疗
恶性肿瘤已成为全球公共医疗的迫切威胁。由于肿瘤的异质性,单一疗法存在很大的局限性,而协同疗法引起了广泛的关注,这表明对共同递送的智能载体的迫切需求。在这项研究中,精心设计了核-壳双金属-有机骨架(MOF)系统,它不仅具有两种材料的独特特性,而且还提供了两个单独的特定功能区来进行联合药物递送。将光敏剂吲哚菁绿(ICG)和化学治疗剂阿霉素(DOX)逐步包封到MIL-88核心和ZIF-8壳的纳米孔中,以构建协同的光热/光动力/化学疗法纳米平台。除了有效的药物输送,MIL-88可以充当纳米马达,将肿瘤微环境中过量的过氧化氢转化成足够的氧气用于光动力疗法。从MIL-88-ICG @ ZIF-8-DOX纳米颗粒释放的DOX在肿瘤酸性微环境下触发,并通过近红外(NIR)光照射进一步加速。的体内抗肿瘤研究显示,与静脉内和瘤内注射纳米颗粒相比,通过将纳米颗粒浓缩成可溶解的微针,具有优异的协同抗肿瘤作用,抑制率明显更高。预计基于双重MOF的多模型协同系统有望在进一步的生物医学应用中发挥作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号