Acta Biomaterialia ( IF 9.4 ) Pub Date : 2020-08-13 , DOI: 10.1016/j.actbio.2020.08.008 Cao Dai Phung 1 , Thanh Tung Pham 1 , Hanh Thuy Nguyen 1 , Tien Tiep Nguyen 1 , Wenquan Ou 1 , Jee-Heon Jeong 1 , Han-Gon Choi 2 , Sae Kwang Ku 3 , Chul Soon Yong 1 , Jong Oh Kim 1
|
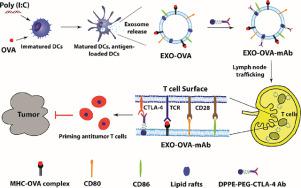
The therapeutic efficacy of current cancer vaccines is far from optimal, mainly because of insufficient induction of antigen-specific T cells and because tumor cells can hijack immunosuppressive mechanisms to evade the immune responses. Generating specific, robust, and long-term immune responses against cancer cells and the attenuating of immunosuppressive factors are critical for effective cancer vaccination. Recently, the engineering of exosomes specifically bind to T cells, and then stimulating tumor-specific T-cell immune responses has emerged as a potential alternative strategy for cancer vaccination. In this study, we generated a bifunctional exosome combining the strategy of vaccination and checkpoint blockade. Exosomes prepared from Ovalbumin (OVA)-pulsed, activated dendritic cells were modified with anti-CTLA-4 antibody (EXO-OVA-mAb) to block this inhibitory molecule and to enhance the specificity of the exosomes toward T cells. Our study provides a unique strategy for functionalizing exosome membrane with anti-CTLA-4 antibody via lipid-anchoring method to synergize efficacy of cancer vaccination and immune checkpoint blockade against the tumor.
Statement of Significance
We designed T-cell-targeting exosomes (EXO-OVA-mAb) decorated with costimulatory molecules, MHCs, antigenic OVA peptide, and anti-CTLA-4 antibody, combining the strategies of vaccines and checkpoint blockade. The exosomes showed enhanced binding to T cells in tumor-draining lymph nodes, effectively induced T-cell activation, and improved the tumor homing of effector T cells, ultimately significantly restraining tumor growth. Thus, EXO-OVA-mAb greatly facilitates T-cell targeting, induces a strong tumor-specific T-cell response, and increased the ratio of effector T cells/regulatory T cells within tumors, resulting in appreciable tumor growth inhibition.
中文翻译:

抗CTLA-4抗体功能化的树突状细胞衍生的外泌体靶向肿瘤引流的淋巴结,可有效诱导抗肿瘤T细胞反应。
当前的癌症疫苗的治疗效果远非最佳,主要是因为对抗原特异性T细胞的诱导不足以及肿瘤细胞可以劫持免疫抑制机制来逃避免疫反应。产生针对癌细胞的特异性,强大和长期的免疫应答以及减弱免疫抑制因子对于有效的癌症疫苗接种至关重要。最近,外泌体的工程改造与T细胞特异性结合,然后刺激肿瘤特异性T细胞免疫应答已成为一种潜在的癌症疫苗替代策略。在这项研究中,我们结合疫苗接种和检查站封锁的策略生成了一个双功能外泌体。由卵清蛋白(OVA)脉冲制备的外泌体,用抗CTLA-4抗体(EXO-OVA-mAb)修饰活化的树突状细胞,以阻断该抑制分子并增强外泌体对T细胞的特异性。我们的研究提供了一种独特的策略,即通过脂质锚定方法使用抗CTLA-4抗体将外泌体膜功能化,以协同增强癌症疫苗接种和针对肿瘤的免疫检查点封锁的功效。
重要声明
我们设计了以共刺激分子,MHC,抗原性OVA肽和抗CTLA-4抗体修饰的靶向T细胞的外来体(EXO-OVA-mAb),并结合了疫苗和关卡封锁策略。外泌体在引流肿瘤的淋巴结中显示出与T细胞的结合增强,有效诱导T细胞活化,并改善了效应T细胞的肿瘤归巢,最终显着抑制了肿瘤的生长。因此,EXO-OVA-mAb极大地促进了T细胞的靶向作用,诱导了强烈的肿瘤特异性T细胞反应,并增加了肿瘤内效应T细胞/调节性T细胞的比例,从而明显抑制了肿瘤的生长。











































 京公网安备 11010802027423号
京公网安备 11010802027423号