Acta Biomaterialia ( IF 9.4 ) Pub Date : 2020-08-13 , DOI: 10.1016/j.actbio.2020.08.007 Yaping Zhong 1 , Yibiao Zou 2 , Lingyan Liu 2 , Ruohan Li 2 , Fengfeng Xue 2 , Tao Yi 3
|
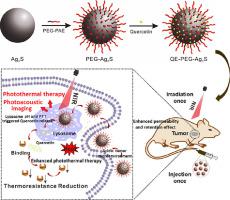
Heat-treated cancer cells have thermo-resistance due to the up-regulated levels of heat shock proteins (HSP) resulting in low therapeutic efficiency and ineffective ablation of tumors. In this work, we report pH-responsive Ag2S nanodots (Ag2S NDs) loaded with HSP70 inhibitor (QE-PEG-Ag2S) for enhanced photothermal cancer therapy. QE-PEG-Ag2S was easily prepared via self-assembly of hydrophobic Ag2S NDs, amphiphilic pH-responsive PEG5k-PAE10k polymer, and an HSP70 inhibitor quercetin (QE). QE-PEG-Ag2S has ideal water-solubility and biocompatibility, can rapidly enter cells, and preferentially accumulate in cell lysosomes. The slightly acidic environment of tumor cells and the acidity of lysosomes as well as the high temperature generated by photothermal therapy under irradiation of NIR light (808 nm) promote the release of the inhibitor molecules to reduce the heat resistance of cancer cells and improve the in vivo photothermal therapy efficiency. Moreover, QE-PEG-Ag2S has good photoacoustic imaging (PAI) ability; this QE-PEG-Ag2S concentration dependent signal can precisely follow the accumulation of the nanomaterials in tumors and dictate the correct time for light therapy. As a result, QE-PEG-Ag2S achieved complete tumor ablation effect with no recurrence when only irradiated with NIR light for 10 min. This approach offers a new approach for the theranostic applications of Ag2S NDs.
Statement of Significance
In this work, pH-responsive Ag2S nanodots loaded with the heat shock protein inhibitor for enhanced photothermal cancer therapy have been simply prepared via self-assembly process. This nanoagent possesses ideal water-solubility and biocompatibility, can rapidly enter cells, and preferentially accumulate in cell lysosomes. The acidic environment of tumor cells and the acidity of lysosomes, as well as the high temperature generated by photothermal therapy under irradiation of NIR light promote the release of the inhibitor molecules from the nanoagent to improve the in vivo photothermal therapy efficiency. Moreover, the photoacoustic imaging (PAI) of the nanoagent can precisely follow the accumulation of the nanomaterials in tumors and dictate the light therapy time to guarantee the complete tumor ablation effect with no recurrence.
中文翻译:

pH响应的Ag2S纳米点负载了热激蛋白70抑制剂,用于光声成像引导的光热癌症治疗。
热处理过的癌细胞由于热休克蛋白(HSP)的水平上调而具有耐热性,从而导致低治疗效率和无效的肿瘤消融。在这项工作中,我们报告了负载HSP70抑制剂(QE-PEG-Ag 2 S)的pH响应性Ag 2 S纳米点(Ag 2 S NDs),用于增强的光热癌症治疗。QE-PEG-的Ag 2 S为容易通过自组装疏水的Ag的制备2小号的ND,两亲性pH响应PEG 5K -PAE 10K聚合物和HSP70抑制剂槲皮素(QE)。QE-PEG-银2S具有理想的水溶性和生物相容性,可以迅速进入细胞,并优先在细胞溶酶体中积累。肿瘤细胞的略微酸性环境和溶酶体的酸度以及NIR光(808纳米)促进所述抑制剂分子的释放,以减少癌细胞的耐热性和提高的照射下由光热治疗产生的高温中体内光热疗法的效率。此外,QE-PEG-Ag 2 S具有良好的光声成像(PAI)能力;这种QE-PEG-Ag 2 S浓度依赖性信号可以精确地跟踪纳米材料在肿瘤中的积累,并决定光疗的正确时间。结果,QE-PEG-Ag 2仅用NIR光照射10分钟,S达到了完全的肿瘤消融效果,没有复发。这种方法为Ag 2 S ND的治疗学应用提供了一种新方法。
重要声明
在这项工作中,通过自组装过程简单地制备了pH响应的Ag 2 S纳米点,其中负载了用于增强光热癌症治疗的热激蛋白抑制剂。该纳米剂具有理想的水溶性和生物相容性,可以迅速进入细胞,并优先在细胞溶酶体中积累。肿瘤细胞的酸性环境和溶酶体的酸性,以及在近红外光照射下通过光热疗法产生的高温,促进了抑制剂分子从纳米剂的释放,从而改善了体内光热疗法的效率。此外,纳米剂的光声成像(PAI)可以精确地跟踪纳米材料在肿瘤中的积累,并决定光疗时间,以确保完全的消融效果而不会复发。









































 京公网安备 11010802027423号
京公网安备 11010802027423号