Frontiers of Environmental Science & Engineering ( IF 6.1 ) Pub Date : 2020-08-09 , DOI: 10.1007/s11783-020-1311-4 Karla Ilić Đurđić , Raluca Ostafe , Olivera Prodanović , Aleksandra Đurđević Đelmaš , Nikolina Popović , Rainer Fischer , Stefan Schillberg , Radivoje Prodanović
|
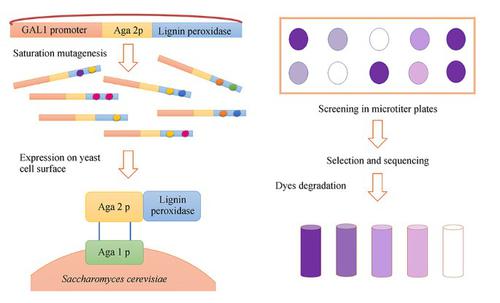
The enzymatic degradation of azo dyes is a promising alternative to ineffective chemical and physical remediation methods. Lignin peroxidase (LiP) from Phanerochaete chrysosporium is a heme-containing lignin-degrading oxidoreductase that catalyzes the peroxide-dependent oxidation of diverse molecules, including industrial dyes. This enzyme is therefore ideal as a starting point for protein engineering. Accordingly, we subjected two positions (165 and 264) in the environment of the catalytic Trp171 residue to saturation mutagenesis, and the resulting library of 104 independent clones was expressed on the surface of yeast cells. This yeast display library was used for the selection of variants with the ability to break down structurally-distinct azo dyes more efficiently. We identified mutants with up to 10-fold greater affinity than wild-type LiP for three diverse azo dyes (Evans blue, amido black 10B and Guinea green) and up to 13-fold higher catalytic activity. Additionally, cell wall fragments displaying mutant LiP enzymes were prepared by toluene-induced cell lysis, achieving significant increases in both enzyme activity and stability compared to a whole-cell biocatalyst. LiP-coated cell wall fragments retained their initial dye degradation activity after 10 reaction cycles each lasting 8 h. The best-performing mutants removed up to 2.5-fold more of each dye than the wild-type LiP in multiple reaction cycles.
中文翻译:

木质素过氧化物酶在催化口袋附近的两个位点发生诱变和应用过氧化物酶包被的酵母细胞壁诱变后,偶氮染料的降解得到改善
偶氮染料的酶促降解是无效的化学和物理修复方法的有希望的替代方法。来自Phanerochaete chrysosporium的木质素过氧化物酶(LiP)是一种含血红素的木质素降解氧化还原酶,可催化包括工业染料在内的各种分子的过氧化物依赖性氧化。因此,该酶是理想的蛋白质工程起点。因此,我们对催化性Trp171残基环境中的两个位置(165和264)进行了饱和诱变,并生成了10 4在酵母细胞表面表达独立的克隆。该酵母展示文库用于选择具有更有效分解结构不同的偶氮染料的能力的变体。我们确定了三种不同的偶氮染料(伊文思蓝,酰胺黑10B和几内亚绿)的亲和力比野生型LiP高10倍,且催化活性高13倍的突变体。另外,通过甲苯诱导的细胞裂解制备了显示突变LiP酶的细胞壁片段,与全细胞生物催化剂相比,酶活性和稳定性均显着提高。涂覆LiP的细胞壁片段在10个反应周期(每个持续8小时)后保留了其初始染料降解活性。表现最佳的突变体最多可去除2个。











































 京公网安备 11010802027423号
京公网安备 11010802027423号