当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New Light on the Mechanism of Phototransduction in Phototropin.
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-13 , DOI: 10.1021/acs.biochem.0c00324 L Henry 1 , O Berntsson 1, 2 , M R Panman 1 , A Cellini 1 , A J Hughes 1 , I Kosheleva 3 , R Henning 3 , S Westenhoff 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-13 , DOI: 10.1021/acs.biochem.0c00324 L Henry 1 , O Berntsson 1, 2 , M R Panman 1 , A Cellini 1 , A J Hughes 1 , I Kosheleva 3 , R Henning 3 , S Westenhoff 1
Affiliation
|
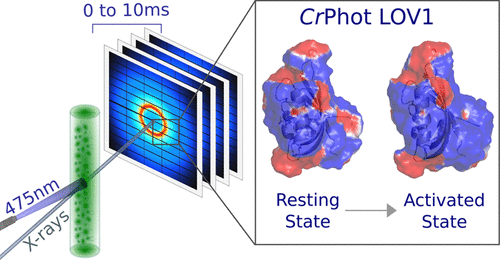
Phototropins are photoreceptor proteins that regulate blue light-dependent biological processes for efficient photosynthesis in plants and algae. The proteins consist of a photosensory domain that responds to the ambient light and an output module that triggers cellular responses. The photosensory domain of phototropin from Chlamydomonas reinhardtii contains two conserved LOV (light–oxygen–voltage) domains with flavin chromophores. Blue light triggers the formation of a covalent cysteine–flavin adduct and upregulates the phototropin kinase activity. Little is known about the structural mechanism that leads to kinase activation and how the two LOV domains contribute to this. Here, we investigate the role of the LOV1 domain from C. reinhardtii phototropin by characterizing the structural changes occurring after blue light illumination with nano- to millisecond time-resolved X-ray solution scattering. By structurally fitting the data with atomic models generated by molecular dynamics simulations, we find that adduct formation induces a rearrangement of the hydrogen bond network from the buried chromophore to the protein surface. In particular, the change in conformation and the associated hydrogen bonding of the conserved glutamine 120 induce a global movement of the β-sheet, ultimately driving a change in the electrostatic potential on the protein surface. On the basis of the change in the electrostatics, we propose a structural model of how LOV1 and LOV2 domains interact and regulate the full-length phototropin from C. reinhardtii. This provides a rationale for how LOV photosensor proteins function and contributes to the optimal design of optogenetic tools based on LOV domains.
中文翻译:

关于 Phototropin 中光转导机制的新见解。
Phototropins 是光感受器蛋白质,可调节蓝光依赖性生物过程,以在植物和藻类中进行有效的光合作用。这些蛋白质由一个响应环境光的光感域和一个触发细胞响应的输出模块组成。莱茵衣藻的光敏素的光敏结构域包含两个带有黄素发色团的保守 LOV(光-氧-电压)结构域。蓝光触发形成共价半胱氨酸 - 黄素加合物并上调促光素激酶活性。关于导致激酶激活的结构机制以及两个 LOV 域如何促成这一点,我们知之甚少。在这里,我们研究了莱茵衣藻LOV1 域的作用通过用纳秒到毫秒时间分辨的 X 射线溶液散射表征蓝光照射后发生的结构变化,向光性素。通过将数据与分子动力学模拟生成的原子模型进行结构拟合,我们发现加合物的形成会导致氢键网络从隐藏的生色团到蛋白质表面的重排。特别是,保守的谷氨酰胺 120 的构象变化和相关的氢键引起 β-折叠的整体运动,最终驱动蛋白质表面静电势的变化。在静电变化的基础上,我们提出了 LOV1 和 LOV2 域如何相互作用并调节莱茵衣藻全长向光性素的结构模型. 这为 LOV 光传感器蛋白的功能提供了基本原理,并有助于基于 LOV 域的光遗传学工具的优化设计。
更新日期:2020-09-08
中文翻译:

关于 Phototropin 中光转导机制的新见解。
Phototropins 是光感受器蛋白质,可调节蓝光依赖性生物过程,以在植物和藻类中进行有效的光合作用。这些蛋白质由一个响应环境光的光感域和一个触发细胞响应的输出模块组成。莱茵衣藻的光敏素的光敏结构域包含两个带有黄素发色团的保守 LOV(光-氧-电压)结构域。蓝光触发形成共价半胱氨酸 - 黄素加合物并上调促光素激酶活性。关于导致激酶激活的结构机制以及两个 LOV 域如何促成这一点,我们知之甚少。在这里,我们研究了莱茵衣藻LOV1 域的作用通过用纳秒到毫秒时间分辨的 X 射线溶液散射表征蓝光照射后发生的结构变化,向光性素。通过将数据与分子动力学模拟生成的原子模型进行结构拟合,我们发现加合物的形成会导致氢键网络从隐藏的生色团到蛋白质表面的重排。特别是,保守的谷氨酰胺 120 的构象变化和相关的氢键引起 β-折叠的整体运动,最终驱动蛋白质表面静电势的变化。在静电变化的基础上,我们提出了 LOV1 和 LOV2 域如何相互作用并调节莱茵衣藻全长向光性素的结构模型. 这为 LOV 光传感器蛋白的功能提供了基本原理,并有助于基于 LOV 域的光遗传学工具的优化设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号