Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Determination of the chiral status of different novel psychoactive substance classes by capillary electrophoresis and β-cyclodextrin derivatives.
Chirality ( IF 2.8 ) Pub Date : 2020-07-15 , DOI: 10.1002/chir.23268 Johannes S Hägele 1 , Eva-Maria Hubner 1 , Martin G Schmid 1
Chirality ( IF 2.8 ) Pub Date : 2020-07-15 , DOI: 10.1002/chir.23268 Johannes S Hägele 1 , Eva-Maria Hubner 1 , Martin G Schmid 1
Affiliation
|
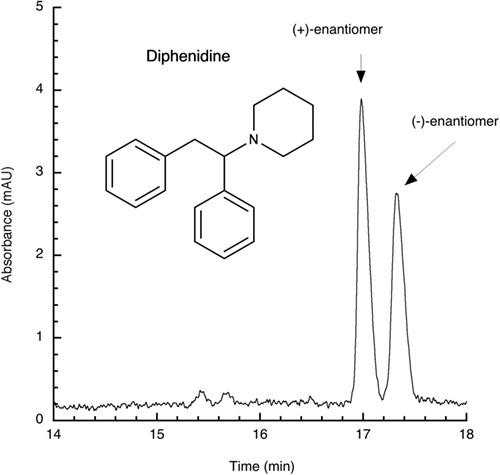
Besides the abuse of well‐known illicit drugs, consumers discovered new synthetic compounds with similar effects but minor alterations in their chemical structure. Originally, these so‐called novel psychoactive substances (NPS) have been created to circumvent law of prosecution because of illicit drug abuse. During the past decade, such compounds came up in generations, the most popular compound was a synthetic cathinone derivative named mephedrone. Cathinones are structurally related to amphetamines; to date, more than 120 completely new derivatives have been synthesized and are traded via the Internet. Cathinones possess a chiral center; however, only little is known about the pharmacology of their enantiomers. However, NPS comprise further chiral compound classes such as amphetamine derivatives, ketamines, 2‐(aminopropyl)benzofurans, and phenidines. In continuation of our project, a cheap and easy‐to‐perform chiral capillary zone electrophoresis method for enantioseparation of cathinones presented previously was extended to the aforementioned compound classes. Enantioresolution was achieved by simply adding native β‐cyclodextrin, acetyl‐β‐cyclodextrin, 2‐hydroxypropyl‐β‐cyclodextrin, or carboxymethyl‐β‐cyclodextrin as chiral selector additives to the background electrolyte. Fifty‐one chiral NPS served as analytes mainly purchased from online vendors via the Internet. Using 10 mM of the aforementioned β‐cyclodextrins in a 10 mM sodium phosphate buffer (pH 2.5), overall, 50 of 51 NPS were resolved. However, chiral separation ability of the selectors differed depending on the analyte. Additionally, simultaneous enantioseparations, the determination of enantiomeric migration orders of selected analytes, and a repeatability study were performed successfully. It was proven that all separated NPS were traded as racemic mixtures.
中文翻译:

通过毛细管电泳和β-环糊精衍生物测定不同新型精神活性物质类别的手性状态。
除了滥用众所周知的非法药物外,消费者还发现了新的合成化合物,它们具有相似的作用,但化学结构发生了微小变化。最初,创建这些所谓的新型精神活性物质(NPS)是为了规避因滥用毒品而引起的起诉法。在过去的十年中,此类化合物世代相传,最流行的化合物是合成的甲基吡啶酮衍生物,称为甲氧麻黄酮。卡西酮在结构上与苯丙胺有关;迄今为止,已经合成了120多种全新的衍生物,并通过Internet进行了交易。卡西酮具有手性中心。然而,关于其对映体的药理学知之甚少。但是,NPS还包含其他手性化合物,例如苯丙胺衍生物,氯胺酮,2-(氨基丙基)苯并呋喃,和吩啶。在我们的项目继续中,以前介绍的一种廉价,易于执行的手性毛细管电泳区分离卡西酮的方法已扩展到上述化合物类别。通过将天然β-环糊精,乙酰基-β-环糊精,2-羟丙基-β-环糊精或羧甲基-β-环糊精作为手性选择剂添加到背景电解质中,即可实现对映体拆分。51个手性NPS用作分析物,主要是通过Internet从在线供应商处购买的。在10 mM磷酸钠缓冲液(pH 2.5)中使用10 mM的上述β-环糊精,总共可分离51 NPS中的50。但是,选择物的手性分离能力根据分析物而不同。此外,同时进行对映体分离 确定所选分析物的对映体迁移顺序,并成功进行了重复性研究。事实证明,所有分离出的NPS都是外消旋混合物。
更新日期:2020-07-15
中文翻译:

通过毛细管电泳和β-环糊精衍生物测定不同新型精神活性物质类别的手性状态。
除了滥用众所周知的非法药物外,消费者还发现了新的合成化合物,它们具有相似的作用,但化学结构发生了微小变化。最初,创建这些所谓的新型精神活性物质(NPS)是为了规避因滥用毒品而引起的起诉法。在过去的十年中,此类化合物世代相传,最流行的化合物是合成的甲基吡啶酮衍生物,称为甲氧麻黄酮。卡西酮在结构上与苯丙胺有关;迄今为止,已经合成了120多种全新的衍生物,并通过Internet进行了交易。卡西酮具有手性中心。然而,关于其对映体的药理学知之甚少。但是,NPS还包含其他手性化合物,例如苯丙胺衍生物,氯胺酮,2-(氨基丙基)苯并呋喃,和吩啶。在我们的项目继续中,以前介绍的一种廉价,易于执行的手性毛细管电泳区分离卡西酮的方法已扩展到上述化合物类别。通过将天然β-环糊精,乙酰基-β-环糊精,2-羟丙基-β-环糊精或羧甲基-β-环糊精作为手性选择剂添加到背景电解质中,即可实现对映体拆分。51个手性NPS用作分析物,主要是通过Internet从在线供应商处购买的。在10 mM磷酸钠缓冲液(pH 2.5)中使用10 mM的上述β-环糊精,总共可分离51 NPS中的50。但是,选择物的手性分离能力根据分析物而不同。此外,同时进行对映体分离 确定所选分析物的对映体迁移顺序,并成功进行了重复性研究。事实证明,所有分离出的NPS都是外消旋混合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号