Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Natural dolapyrrolidone: Isolation and absolute stereochemistry of a substructure of bioactive peptides.
Chirality ( IF 2.8 ) Pub Date : 2020-06-30 , DOI: 10.1002/chir.23264 Ayana Murakami 1 , Jun-Ichi Hayashi 2 , Kazunobu Igawa 2, 3 , Miki Tsutsumi 4 , Katsuhiko Tomooka 2, 3 , Hiroshi Nagai 4 , Tatsuo Nehira 1, 5
Chirality ( IF 2.8 ) Pub Date : 2020-06-30 , DOI: 10.1002/chir.23264 Ayana Murakami 1 , Jun-Ichi Hayashi 2 , Kazunobu Igawa 2, 3 , Miki Tsutsumi 4 , Katsuhiko Tomooka 2, 3 , Hiroshi Nagai 4 , Tatsuo Nehira 1, 5
Affiliation
|
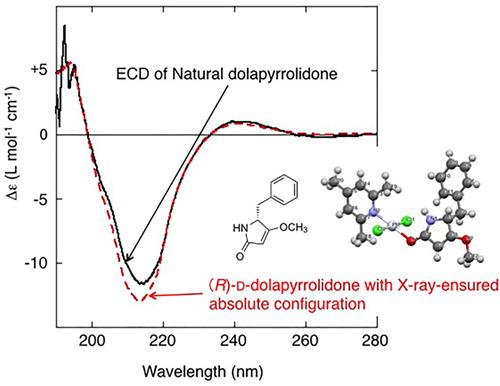
During the course of our chemical analysis of the hydrophilic fractions from marine cyanobacterium Moorena producens, we have isolated natural dolapyrrolidone (Dpy, 1), a natural pyrrolidone derived from phenylalanine, for the first time as a single compound. Compound 1, with an (S)‐l absolute stereochemistry, was previously identified as a substructure that is common among several bioactive natural peptides. Surprisingly, the absolute stereochemistry of the isolated natural 1, determined through total synthesis, was (R)‐d. This result was unambiguously determined by HPLC analysis using a chiral stationary column by comparing the retention times of the natural 1 and authentic samples of synthetic enantiomers. To verify the unexpected result, the absolute stereochemistry of the natural 1 was confirmed by X‐ray crystallographic analysis of Pt‐complex derivative using the synthetic enantiomer.
中文翻译:

天然多拉吡咯烷酮:生物活性肽亚结构的分离和绝对立体化学。
在对海洋蓝藻穆氏藻生产物中的亲水性部分进行化学分析的过程中,我们首次分离出天然多吡咯烷酮(Dpy,1),这是一种衍生自苯丙氨酸的天然吡咯烷酮,为单一化合物。化合物1,与(小号) -升的绝对立体化学,先前被确定为一个子结构是共同的几个生物活性天然肽中。出乎意料的是,通过全合成确定的分离出的天然1的绝对立体化学为(R)-d。通过比较手性固定对映体的天然1和真实样品的保留时间,使用手性固定柱通过HPLC分析明确确定了该结果。为了验证出乎意料的结果,使用合成对映异构体对Pt-络合物衍生物进行X射线晶体学分析证实了天然1的绝对立体化学。
更新日期:2020-06-30
中文翻译:

天然多拉吡咯烷酮:生物活性肽亚结构的分离和绝对立体化学。
在对海洋蓝藻穆氏藻生产物中的亲水性部分进行化学分析的过程中,我们首次分离出天然多吡咯烷酮(Dpy,1),这是一种衍生自苯丙氨酸的天然吡咯烷酮,为单一化合物。化合物1,与(小号) -升的绝对立体化学,先前被确定为一个子结构是共同的几个生物活性天然肽中。出乎意料的是,通过全合成确定的分离出的天然1的绝对立体化学为(R)-d。通过比较手性固定对映体的天然1和真实样品的保留时间,使用手性固定柱通过HPLC分析明确确定了该结果。为了验证出乎意料的结果,使用合成对映异构体对Pt-络合物衍生物进行X射线晶体学分析证实了天然1的绝对立体化学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号