Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
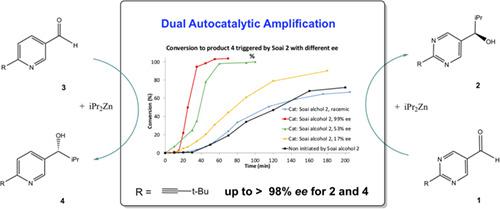
Kinetic relationship in parallel autocatalytic amplifications of pyridyl alkanol and chiral trigger pyrimidyl alkanol.
Chirality ( IF 2.8 ) Pub Date : 2020-06-30 , DOI: 10.1002/chir.23256 Carlo Romagnoli 1 , Bora Sieng 1 , Mohamed Amedjkouh 1
Chirality ( IF 2.8 ) Pub Date : 2020-06-30 , DOI: 10.1002/chir.23256 Carlo Romagnoli 1 , Bora Sieng 1 , Mohamed Amedjkouh 1
Affiliation
|
Experimental and kinetic analysis of a chemical system combines autocatalytic amplification of 2‐alkynyl‐5‐pyrimidyl alkanol 2 and 6‐alkynyl‐3‐pyridyl akanol 4 in which 2 acts as a chiral trigger and 4 being the subsequent autocatalyst. Starting from a very low initial ee, both alkanols are produced with high enantiopurity in one single cycle. This provides insight into a dual nonlinear amplification of chirality observed with amplifying trigger 2 and accelerated amplification of autocatalyst 4. These kinetic studies reveal a five‐fold magnitude superior amplification rates of 4 associated with trigger's enantiopurity at the outset.
中文翻译:

吡啶烷醇和手性引发嘧啶烷醇的平行自催化扩增中的动力学关系。
实验和链烷醇2-炔基-5-嘧啶基的化学系统结合自催化扩增的动力学分析2和6 -炔基-3-吡啶基akanol 4,其中2只充当手性触发和4是所述后续的尾气净化催化剂。从非常低的初始ee开始,两种链烷醇都在一个单一循环中以高对映体纯度生产。这提供了对手性的双重非线性扩增的见解,该双重非线性是通过放大触发2和加速自动催化剂4观察到的。这些动力学研究表明,放大倍数为4的五倍。 一开始与扳机的对映体有关。
更新日期:2020-06-30
中文翻译:

吡啶烷醇和手性引发嘧啶烷醇的平行自催化扩增中的动力学关系。
实验和链烷醇2-炔基-5-嘧啶基的化学系统结合自催化扩增的动力学分析2和6 -炔基-3-吡啶基akanol 4,其中2只充当手性触发和4是所述后续的尾气净化催化剂。从非常低的初始ee开始,两种链烷醇都在一个单一循环中以高对映体纯度生产。这提供了对手性的双重非线性扩增的见解,该双重非线性是通过放大触发2和加速自动催化剂4观察到的。这些动力学研究表明,放大倍数为4的五倍。 一开始与扳机的对映体有关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号