当前位置:
X-MOL 学术
›
J. Cell. Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alkaline phosphatase dual-binding sites for collagen dictate cell migration and microvessel assembly in vitro.
Journal of Cellular Biochemistry ( IF 3.0 ) Pub Date : 2020-08-03 , DOI: 10.1002/jcb.29835 Susana G Guerreiro 1, 2, 3, 4 , Ronald E Unger 5 , Nuno M F S A Cerqueira 6 , Anne Sartoris 5 , Maria J Martins 1, 4 , Mário A Barbosa 1, 2, 7 , Raquel Soares 1, 4 , Pedro L Granja 1, 2, 3, 7 , Charles J Kirkpatrick 5
Journal of Cellular Biochemistry ( IF 3.0 ) Pub Date : 2020-08-03 , DOI: 10.1002/jcb.29835 Susana G Guerreiro 1, 2, 3, 4 , Ronald E Unger 5 , Nuno M F S A Cerqueira 6 , Anne Sartoris 5 , Maria J Martins 1, 4 , Mário A Barbosa 1, 2, 7 , Raquel Soares 1, 4 , Pedro L Granja 1, 2, 3, 7 , Charles J Kirkpatrick 5
Affiliation
|
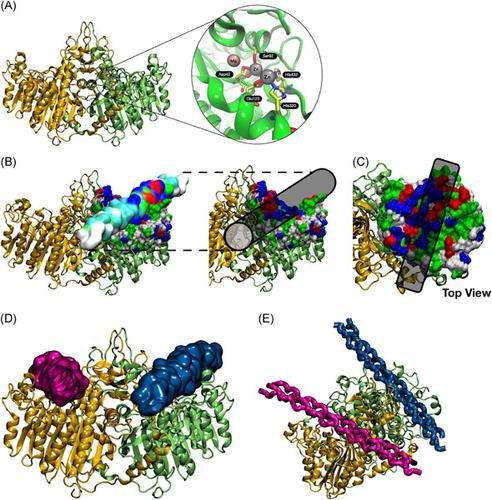
Interactions between cell types, growth factors, and extracellular matrix components involved in angiogenesis are crucial for new vessel formation leading to tissue regeneration. This study investigated whether cocultures of fibroblasts and endothelial cells (ECs; from macro‐ or microvasculature) play a role in the formation of microvessel‐like structures by ECs, as well as modulate fibroblast differentiation and growth factors production (vascular endothelial cell growth factor, basic fibroblast growth factor, active transforming growth factor‐β1, and interleukin‐8), which are important for vessel sprouting and maturation. Data obtained revealed that in vitro coculture systems of fibroblasts and human ECs stimulate collagen synthesis and growth factors production by fibroblasts that ultimately affect the formation and distribution of microvessel‐like structures in cell cultures. In this study, areas with activated fibroblasts and high alkaline phosphatase (ALP) activity were also observed in cocultures. Molecular docking assays revealed that ALP has two binding positions for collagen, suggesting its impact in collagen proteins’ aggregation, cell migration, and microvessel assembly. These findings indicate that bioinformatics and coculture systems are complementary tools for investigating the participation of proteins, like collagen and ALP in angiogenesis.
中文翻译:

胶原蛋白的碱性磷酸酶双结合位点决定了细胞迁移和体外微血管组装。
涉及血管生成的细胞类型,生长因子和细胞外基质成分之间的相互作用对于导致组织再生的新血管形成至关重要。这项研究调查了成纤维细胞和内皮细胞(EC;来自大血管或微脉管系统)的共培养是否在EC形成微血管样结构中发挥作用,以及调节成纤维细胞分化和生长因子产生(血管内皮细胞生长因子,碱性成纤维细胞生长因子,活性转化生长因子-β1和白介素-8),它们对于血管发芽和成熟很重要。获得的数据表明,成纤维细胞和人EC的体外共培养系统刺激成纤维细胞产生胶原蛋白合成和生长因子,最终影响细胞培养物中微血管样结构的形成和分布。在这项研究中,在共培养物中还观察到具有激活的成纤维细胞和高碱性磷酸酶(ALP)活性的区域。分子对接分析显示,ALP具有两个与胶原蛋白的结合位置,表明其对胶原蛋白的聚集,细胞迁移和微血管组装的影响。这些发现表明,生物信息学和共培养系统是用于研究诸如胶原蛋白和ALP的蛋白质在血管生成中的参与的补充工具。在共培养物中还观察到具有活化的成纤维细胞和高碱性磷酸酶(ALP)活性的区域。分子对接分析显示,ALP具有两个与胶原蛋白的结合位置,表明其对胶原蛋白的聚集,细胞迁移和微血管组装的影响。这些发现表明,生物信息学和共培养系统是用于研究诸如胶原蛋白和ALP的蛋白质在血管生成中的参与的补充工具。在共培养中也观察到具有活化的成纤维细胞和高碱性磷酸酶(ALP)活性的区域。分子对接分析显示,ALP具有两个与胶原蛋白的结合位置,表明其对胶原蛋白的聚集,细胞迁移和微血管组装的影响。这些发现表明,生物信息学和共培养系统是用于研究诸如胶原蛋白和ALP的蛋白质在血管生成中的参与的补充工具。
更新日期:2020-08-03
中文翻译:

胶原蛋白的碱性磷酸酶双结合位点决定了细胞迁移和体外微血管组装。
涉及血管生成的细胞类型,生长因子和细胞外基质成分之间的相互作用对于导致组织再生的新血管形成至关重要。这项研究调查了成纤维细胞和内皮细胞(EC;来自大血管或微脉管系统)的共培养是否在EC形成微血管样结构中发挥作用,以及调节成纤维细胞分化和生长因子产生(血管内皮细胞生长因子,碱性成纤维细胞生长因子,活性转化生长因子-β1和白介素-8),它们对于血管发芽和成熟很重要。获得的数据表明,成纤维细胞和人EC的体外共培养系统刺激成纤维细胞产生胶原蛋白合成和生长因子,最终影响细胞培养物中微血管样结构的形成和分布。在这项研究中,在共培养物中还观察到具有激活的成纤维细胞和高碱性磷酸酶(ALP)活性的区域。分子对接分析显示,ALP具有两个与胶原蛋白的结合位置,表明其对胶原蛋白的聚集,细胞迁移和微血管组装的影响。这些发现表明,生物信息学和共培养系统是用于研究诸如胶原蛋白和ALP的蛋白质在血管生成中的参与的补充工具。在共培养物中还观察到具有活化的成纤维细胞和高碱性磷酸酶(ALP)活性的区域。分子对接分析显示,ALP具有两个与胶原蛋白的结合位置,表明其对胶原蛋白的聚集,细胞迁移和微血管组装的影响。这些发现表明,生物信息学和共培养系统是用于研究诸如胶原蛋白和ALP的蛋白质在血管生成中的参与的补充工具。在共培养中也观察到具有活化的成纤维细胞和高碱性磷酸酶(ALP)活性的区域。分子对接分析显示,ALP具有两个与胶原蛋白的结合位置,表明其对胶原蛋白的聚集,细胞迁移和微血管组装的影响。这些发现表明,生物信息学和共培养系统是用于研究诸如胶原蛋白和ALP的蛋白质在血管生成中的参与的补充工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号