Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spectral study on inclusion interaction and enantiorecognition of 2-aryl carboxylic acids with hydroxypropyl-β-cyclodextrin.
Chirality ( IF 2.8 ) Pub Date : 2020-08-03 , DOI: 10.1002/chir.23276 Yang Jin 1 , Wenyu Sun 1 , Huawei Lv 1 , Shengqiang Tong 1
Chirality ( IF 2.8 ) Pub Date : 2020-08-03 , DOI: 10.1002/chir.23276 Yang Jin 1 , Wenyu Sun 1 , Huawei Lv 1 , Shengqiang Tong 1
Affiliation
|
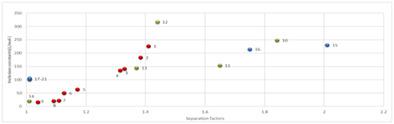
The inclusion interaction between hydroxypropyl‐β‐cyclodextrin (HP‐β‐CD) and 21 2‐aryl carboxylic acids was investigated by UV (ultraviolet) spectrophotometer. The inclusion constant of each 2‐aryl carboxylic acids with HP‐β‐CD was determined by Benesi–Hildebrand's equation. According to our previous work, it was found that a high inclusion constant for inclusion complex formed by a racemate and cyclodextrin was always observed with the fact that a high enantioseparation factor was achieved for the racemate in enantioseparation by liquid–liquid chromatography, which suggested that high binding combination between racemate and cyclodextrin is very important for a successful enantioseparation in enantioselective liquid–liquid extraction. Among all the studied subjects, mandelic acid enantiomer, 2,3‐diphenylpropionic acid enantiomer, and naproxen enantiomer were selected for the further study. The inclusion constants of enantiomers of these three subjects were determined by UV spectra, which indicated that a necessary difference in inclusion constants between enantiomer and cyclodextrin was also essential. It was found in UV spectra that the absorbance of the analytes with the addition of cyclodextrin would increase or decrease, which was determined by the type of electron excitation. The conformation changes of small molecules can lead to the changes of chromophore valence electron clouds distribution, causing the HOMO‐LUMO energy difference decreased. Thus, a red shift of the wavelength of the maximum absorption was produced indicating that the possibility of the molecular interaction of enantiomers with HP‐β‐CD exists.
中文翻译:

光谱研究2-芳基羧酸与羟丙基-β-环糊精的包合物相互作用和对映体识别。
用紫外(紫外)分光光度计研究了羟丙基-β-环糊精(HP-β-CD)与21种2-芳基羧酸之间的夹杂作用。通过Benesi-Hildebrand方程确定HP-β-CD中每种2-芳基羧酸的夹杂常数。根据我们以前的工作,发现始终观察到由外消旋体和环糊精形成的包合物的高包合常数,同时通过液-液相色谱对映体的分离得到了高的对映体分离因子,这表明:外消旋物和环糊精之间的高结合力对成功进行对映选择性液-液萃取的对映体分离非常重要。在所有研究对象中,扁桃酸对映体,2,3-二苯基丙酸对映体,选择萘普生对映体和萘普生对映体进行进一步研究。通过紫外光谱确定这三个对象的对映体的包合常数,这表明对映体和环糊精之间的包合常数的必要差异也是必不可少的。在紫外光谱中发现,添加环糊精后分析物的吸光度会增加或降低,这取决于电子激发的类型。小分子的构象变化可导致发色团价电子云分布的变化,从而导致HOMO-LUMO能量差减小。因此,产生了最大吸收波长的红移,表明存在对映异构体与HP-β-CD分子相互作用的可能性。通过紫外光谱确定这三个对象的对映体的包合常数,这表明对映体和环糊精之间的包合常数的必要差异也是必不可少的。在紫外光谱中发现,添加环糊精后分析物的吸光度会增加或降低,这取决于电子激发的类型。小分子的构象变化可导致发色团价电子云分布的变化,从而导致HOMO-LUMO能量差减小。因此,产生了最大吸收波长的红移,表明存在对映异构体与HP-β-CD分子相互作用的可能性。通过紫外光谱确定这三个对象的对映体的包合常数,这表明对映体和环糊精之间的包合常数的必要差异也是必不可少的。在紫外光谱中发现,添加环糊精后分析物的吸光度会增加或降低,这取决于电子激发的类型。小分子的构象变化可导致发色团价电子云分布的变化,从而导致HOMO-LUMO能量差减小。因此,产生了最大吸收波长的红移,表明存在对映异构体与HP-β-CD分子相互作用的可能性。这表明对映异构体和环糊精之间包合常数的必要差异也是必不可少的。在紫外光谱中发现,添加环糊精后分析物的吸光度会增加或降低,这取决于电子激发的类型。小分子的构象变化可导致发色团价电子云分布的变化,从而导致HOMO-LUMO能量差减小。因此,产生了最大吸收波长的红移,表明存在对映异构体与HP-β-CD分子相互作用的可能性。这表明对映异构体和环糊精之间包合常数的必要差异也是必不可少的。在紫外光谱中发现,添加环糊精后分析物的吸光度会增加或降低,这取决于电子激发的类型。小分子的构象变化可导致发色团价电子云分布的变化,从而导致HOMO-LUMO能量差减小。因此,产生了最大吸收波长的红移,表明存在对映异构体与HP-β-CD分子相互作用的可能性。在紫外光谱中发现,添加环糊精后分析物的吸光度会增加或降低,这取决于电子激发的类型。小分子的构象变化可导致发色团价电子云分布的变化,从而导致HOMO-LUMO能量差减小。因此,产生了最大吸收波长的红移,表明存在对映异构体与HP-β-CD分子相互作用的可能性。在紫外光谱中发现,添加环糊精后分析物的吸光度会增加或降低,这取决于电子激发的类型。小分子的构象变化可导致发色团价电子云分布的变化,从而导致HOMO-LUMO能量差减小。因此,产生了最大吸收波长的红移,表明存在对映异构体与HP-β-CD分子相互作用的可能性。
更新日期:2020-08-03
中文翻译:

光谱研究2-芳基羧酸与羟丙基-β-环糊精的包合物相互作用和对映体识别。
用紫外(紫外)分光光度计研究了羟丙基-β-环糊精(HP-β-CD)与21种2-芳基羧酸之间的夹杂作用。通过Benesi-Hildebrand方程确定HP-β-CD中每种2-芳基羧酸的夹杂常数。根据我们以前的工作,发现始终观察到由外消旋体和环糊精形成的包合物的高包合常数,同时通过液-液相色谱对映体的分离得到了高的对映体分离因子,这表明:外消旋物和环糊精之间的高结合力对成功进行对映选择性液-液萃取的对映体分离非常重要。在所有研究对象中,扁桃酸对映体,2,3-二苯基丙酸对映体,选择萘普生对映体和萘普生对映体进行进一步研究。通过紫外光谱确定这三个对象的对映体的包合常数,这表明对映体和环糊精之间的包合常数的必要差异也是必不可少的。在紫外光谱中发现,添加环糊精后分析物的吸光度会增加或降低,这取决于电子激发的类型。小分子的构象变化可导致发色团价电子云分布的变化,从而导致HOMO-LUMO能量差减小。因此,产生了最大吸收波长的红移,表明存在对映异构体与HP-β-CD分子相互作用的可能性。通过紫外光谱确定这三个对象的对映体的包合常数,这表明对映体和环糊精之间的包合常数的必要差异也是必不可少的。在紫外光谱中发现,添加环糊精后分析物的吸光度会增加或降低,这取决于电子激发的类型。小分子的构象变化可导致发色团价电子云分布的变化,从而导致HOMO-LUMO能量差减小。因此,产生了最大吸收波长的红移,表明存在对映异构体与HP-β-CD分子相互作用的可能性。通过紫外光谱确定这三个对象的对映体的包合常数,这表明对映体和环糊精之间的包合常数的必要差异也是必不可少的。在紫外光谱中发现,添加环糊精后分析物的吸光度会增加或降低,这取决于电子激发的类型。小分子的构象变化可导致发色团价电子云分布的变化,从而导致HOMO-LUMO能量差减小。因此,产生了最大吸收波长的红移,表明存在对映异构体与HP-β-CD分子相互作用的可能性。这表明对映异构体和环糊精之间包合常数的必要差异也是必不可少的。在紫外光谱中发现,添加环糊精后分析物的吸光度会增加或降低,这取决于电子激发的类型。小分子的构象变化可导致发色团价电子云分布的变化,从而导致HOMO-LUMO能量差减小。因此,产生了最大吸收波长的红移,表明存在对映异构体与HP-β-CD分子相互作用的可能性。这表明对映异构体和环糊精之间包合常数的必要差异也是必不可少的。在紫外光谱中发现,添加环糊精后分析物的吸光度会增加或降低,这取决于电子激发的类型。小分子的构象变化可导致发色团价电子云分布的变化,从而导致HOMO-LUMO能量差减小。因此,产生了最大吸收波长的红移,表明存在对映异构体与HP-β-CD分子相互作用的可能性。在紫外光谱中发现,添加环糊精后分析物的吸光度会增加或降低,这取决于电子激发的类型。小分子的构象变化可导致发色团价电子云分布的变化,从而导致HOMO-LUMO能量差减小。因此,产生了最大吸收波长的红移,表明存在对映异构体与HP-β-CD分子相互作用的可能性。在紫外光谱中发现,添加环糊精后分析物的吸光度会增加或降低,这取决于电子激发的类型。小分子的构象变化可导致发色团价电子云分布的变化,从而导致HOMO-LUMO能量差减小。因此,产生了最大吸收波长的红移,表明存在对映异构体与HP-β-CD分子相互作用的可能性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号