当前位置:
X-MOL 学术
›
Biopolymers
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Side‐chain thioamides as fluorescence quenching probes
Biopolymers ( IF 3.2 ) Pub Date : 2020-06-17 , DOI: 10.1002/bip.23384 D Miklos Robkis 1 , Eileen M Hoang 2 , Pengse Po 3 , Carol J Deutsch 3 , E James Petersson 1, 2
Biopolymers ( IF 3.2 ) Pub Date : 2020-06-17 , DOI: 10.1002/bip.23384 D Miklos Robkis 1 , Eileen M Hoang 2 , Pengse Po 3 , Carol J Deutsch 3 , E James Petersson 1, 2
Affiliation
|
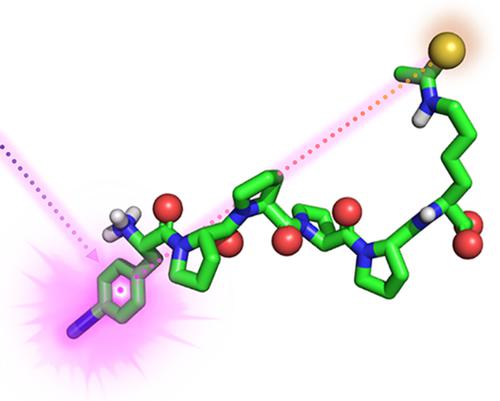
Thioamides, single atom oxygen‐to‐sulfur substitutions of canonical amide bonds, can be valuable probes for protein folding and protease studies. Here, we investigate the fluorescence quenching properties of thioamides incorporated into the side‐chains of amino acids. We synthesize and incorporate Fmoc‐protected, solid‐phase peptide synthesis building blocks for introducing Nε‐thioacetyl‐lysine and γ‐thioasparagine. Using rigid model peptides, we demonstrate the distance‐dependent fluorescence quenching of these thioamides. Furthermore, we describe attempts to incorporate of Nε‐thioacetyl‐lysine into proteins expressed in Escherichia coli using amber codon suppression.
中文翻译:

侧链硫代酰胺作为荧光猝灭探针
硫代酰胺是典型酰胺键的单原子氧硫取代,可以成为蛋白质折叠和蛋白酶研究的有价值的探针。在这里,我们研究了掺入氨基酸侧链的硫代酰胺的荧光猝灭特性。我们合成并整合了 Fmoc 保护的固相肽合成构建模块,用于引入 Nε-硫代乙酰基-赖氨酸和 γ-硫代天冬酰胺。使用刚性模型肽,我们证明了这些硫代酰胺的距离依赖性荧光猝灭。此外,我们描述了使用琥珀密码子抑制将 Nε-硫代乙酰基-赖氨酸整合到大肠杆菌中表达的蛋白质中的尝试。
更新日期:2020-06-17
中文翻译:

侧链硫代酰胺作为荧光猝灭探针
硫代酰胺是典型酰胺键的单原子氧硫取代,可以成为蛋白质折叠和蛋白酶研究的有价值的探针。在这里,我们研究了掺入氨基酸侧链的硫代酰胺的荧光猝灭特性。我们合成并整合了 Fmoc 保护的固相肽合成构建模块,用于引入 Nε-硫代乙酰基-赖氨酸和 γ-硫代天冬酰胺。使用刚性模型肽,我们证明了这些硫代酰胺的距离依赖性荧光猝灭。此外,我们描述了使用琥珀密码子抑制将 Nε-硫代乙酰基-赖氨酸整合到大肠杆菌中表达的蛋白质中的尝试。











































 京公网安备 11010802027423号
京公网安备 11010802027423号