当前位置:
X-MOL 学术
›
J. Cell. Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Retinal proteomics of experimental glaucoma model reveal intraocular pressure-induced mediators of neurodegenerative changes.
Journal of Cellular Biochemistry ( IF 3.0 ) Pub Date : 2020-07-21 , DOI: 10.1002/jcb.29822 Mehdi Mirzaei 1, 2, 3 , Vivek K Gupta 2 , Nitin Chitranshi 2 , Liting Deng , Kanishka Pushpitha 2 , Mojdeh Abbasi 2 , Joel M Chick 4 , Rashi Rajput 2 , Yunqi Wu 1, 3 , Matthew J McKay 1, 3 , Ghasem H Salekdeh 5 , Veer B Gupta 6 , Paul A Haynes 1 , Stuart L Graham 2
Journal of Cellular Biochemistry ( IF 3.0 ) Pub Date : 2020-07-21 , DOI: 10.1002/jcb.29822 Mehdi Mirzaei 1, 2, 3 , Vivek K Gupta 2 , Nitin Chitranshi 2 , Liting Deng , Kanishka Pushpitha 2 , Mojdeh Abbasi 2 , Joel M Chick 4 , Rashi Rajput 2 , Yunqi Wu 1, 3 , Matthew J McKay 1, 3 , Ghasem H Salekdeh 5 , Veer B Gupta 6 , Paul A Haynes 1 , Stuart L Graham 2
Affiliation
|
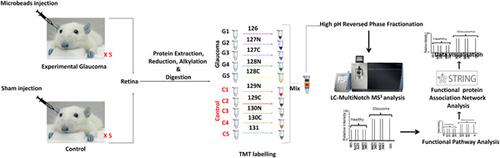
Current evidence suggests that exposure to chronically induced intraocular pressure (IOP) leads to neurodegenerative changes in the inner retina. This study aimed to determine retinal proteomic alterations in a rat model of glaucoma and compared findings with human retinal proteomics changes in glaucoma reported previously. We developed an experimental glaucoma rat model by subjecting the rats to increased IOP (9.3 ± 0.1 vs 20.8 ± 1.6 mm Hg) by weekly microbead injections into the eye (8 weeks). The retinal tissues were harvested from control and glaucomatous eyes and protein expression changes analysed using a multiplexed quantitative proteomics approach (TMT‐MS3). Immunofluorescence was performed for selected protein markers for data validation. Our study identified 4304 proteins in the rat retinas. Out of these, 139 proteins were downregulated (≤0.83) while the expression of 109 proteins was upregulated (≥1.2‐fold change) under glaucoma conditions (P ≤ .05). Computational analysis revealed reduced expression of proteins associated with glutathione metabolism, mitochondrial dysfunction/oxidative phosphorylation, cytoskeleton, and actin filament organisation, along with increased expression of proteins in coagulation cascade, apoptosis, oxidative stress, and RNA processing. Further functional network analysis highlighted the differential modulation of nuclear receptor signalling, cellular survival, protein synthesis, transport, and cellular assembly pathways. Alterations in crystallin family, glutathione metabolism, and mitochondrial dysfunction associated proteins shared similarities between the animal model of glaucoma and the human disease condition. In contrast, the activation of the classical complement pathway and upregulation of cholesterol transport proteins were exclusive to human glaucoma. These findings provide insights into the neurodegenerative mechanisms that are specifically affected in the retina in response to chronically elevated IOP.
中文翻译:

实验性青光眼模型的视网膜蛋白质组学揭示了眼压诱导的神经退行性变化的介质。
目前的证据表明,暴露于长期诱发的眼内压(IOP)会导致视网膜内神经退行性变化。本研究旨在确定青光眼大鼠模型中视网膜蛋白质组的变化,并将结果与之前报道的青光眼中人类视网膜蛋白质组的变化进行比较。我们开发了一种实验性青光眼大鼠模型,每周向眼内注射微珠(8 周),使大鼠的眼压升高(9.3 ± 0.1 与 20.8 ± 1.6 mm Hg)。从对照眼和青光眼眼中采集视网膜组织,并使用多重定量蛋白质组学方法 (TMT-MS3) 分析蛋白质表达变化。对选定的蛋白质标记物进行免疫荧光以进行数据验证。我们的研究在大鼠视网膜中发现了 4304 种蛋白质。其中,在青光眼条件下,139 个蛋白表达下调(≤0.83),而 109 个蛋白表达上调(≥1.2 倍变化)(P ≤ .05)。计算分析显示,与谷胱甘肽代谢、线粒体功能障碍/氧化磷酸化、细胞骨架和肌动蛋白丝组织相关的蛋白质表达减少,同时凝血级联、细胞凋亡、氧化应激和 RNA 加工中蛋白质的表达增加。进一步的功能网络分析强调了核受体信号传导、细胞存活、蛋白质合成、运输和细胞组装途径的差异调节。青光眼动物模型和人类疾病状况之间的晶状体蛋白家族、谷胱甘肽代谢和线粒体功能障碍相关蛋白的改变具有相似性。相比之下,经典补体途径的激活和胆固醇转运蛋白的上调是人类青光眼所独有的。这些发现提供了对视网膜因长期升高的眼压而受到特别影响的神经退行性机制的见解。
更新日期:2020-07-21
中文翻译:

实验性青光眼模型的视网膜蛋白质组学揭示了眼压诱导的神经退行性变化的介质。
目前的证据表明,暴露于长期诱发的眼内压(IOP)会导致视网膜内神经退行性变化。本研究旨在确定青光眼大鼠模型中视网膜蛋白质组的变化,并将结果与之前报道的青光眼中人类视网膜蛋白质组的变化进行比较。我们开发了一种实验性青光眼大鼠模型,每周向眼内注射微珠(8 周),使大鼠的眼压升高(9.3 ± 0.1 与 20.8 ± 1.6 mm Hg)。从对照眼和青光眼眼中采集视网膜组织,并使用多重定量蛋白质组学方法 (TMT-MS3) 分析蛋白质表达变化。对选定的蛋白质标记物进行免疫荧光以进行数据验证。我们的研究在大鼠视网膜中发现了 4304 种蛋白质。其中,在青光眼条件下,139 个蛋白表达下调(≤0.83),而 109 个蛋白表达上调(≥1.2 倍变化)(P ≤ .05)。计算分析显示,与谷胱甘肽代谢、线粒体功能障碍/氧化磷酸化、细胞骨架和肌动蛋白丝组织相关的蛋白质表达减少,同时凝血级联、细胞凋亡、氧化应激和 RNA 加工中蛋白质的表达增加。进一步的功能网络分析强调了核受体信号传导、细胞存活、蛋白质合成、运输和细胞组装途径的差异调节。青光眼动物模型和人类疾病状况之间的晶状体蛋白家族、谷胱甘肽代谢和线粒体功能障碍相关蛋白的改变具有相似性。相比之下,经典补体途径的激活和胆固醇转运蛋白的上调是人类青光眼所独有的。这些发现提供了对视网膜因长期升高的眼压而受到特别影响的神经退行性机制的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号