Current Organic Synthesis ( IF 1.7 ) Pub Date : 2020-10-31 , DOI: 10.2174/1570179417666200628021125 Mohamed Ahmed Mahmoud Abdel Reheim 1 , Ibrahim Saad Abdel Hafiz 1 , Mohamed Ahmed Elian 1
|
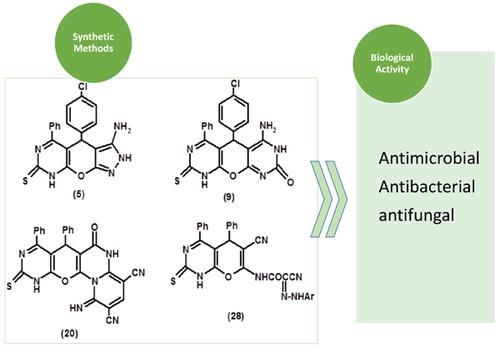
Aim and Objective: A novel collection of fused pyrimidine, pyridine, pyrazole, chromene and thiophene derivatives 2-30 have been newly synthesized by using the 1a, b as starting material. Fused pyrane exhibits a range of pharmacological activity such as cancer agents [1], antimicrobial [2-4], antioxidant [5], antiproliferative [6], cytotoxic activity [7], anticipated antitumor [8], antiparkinsonian [9] and anti-inflammatory [10]. Moreover, pyrane derivatives are well known for bacterial biofilm disruptor [11], anticonvulsant [12] and inhibitors of mycobacterium bovis [13].
Materials and Methods: All melting points were measured using the Akofler Block instrument and are uncorrected. IR spectra (KBr) were recorded on a FTIR 5300 spectrometer (υ, cm-1). The 1H-NMR spectra were recorded on a Varian Gemini spectrometer. The 1H-NMR spectra were run at 300, 400 MHz and 13C-NMR spectra were run at 100 MHz in DMSO-d6, CDCl3 as solvents. The chemical shifts are expressed in parts per million (ppm) by using tetramethylsilane (TMS) as an internal reference, 1000 EX mass spectrometer at 70 eV. The purity of synthesized compounds was checked by thin-layer chromatography (TLC) (aluminum sheets) using nhexane, EtOAc (9:1, V/V, 7:3 V/V) eluent. Elemental analyses were carried out by the Microanalytical Research Center, Faculty of Science, and Microanalytical Unit, Faculty of Pharmacy, Cairo University, Egypt.
Results and Discussion: A novel series of azoles and azines were designed and prepared via the reaction of 7-amino- 5-(4-chlorophenyl)-4-phenyl-2-thioxo-2,5-dihydro-1H-pyrano- [2,3-d]pyrimidine-6-carbonitrile 1a and 7-amino-4,5- diphenyl-2-thioxo-2,5-dihydro-1H-pyrano[2,3-d]-pyrimidine-6-carbonitrile 1b with some electrophilic and nucleophilic reagents. The structures of target compounds were confirmed by elemental analyses and spectral data. The novel synthesized compounds showed good antimicrobial activity against the previously mentioned microorganisms.
Conclusion: In conclusion, compounds 1a, 1b underwent ready cyclization to give fused heterocyclic compounds through reaction with different reagents and under different conditions and subjected to antimicrobial screening.
中文翻译:

带有嘧啶丁硫酮部分的一些新型嘧啶,吡唑,二甲苯和四氢苯并[b]噻吩衍生物的合成及抗菌评价。
目的与目的:以1a,b为起始原料,新合成了融合的嘧啶,吡啶,吡唑,色烯和噻吩衍生物2-30。熔融的pyr烷表现出一系列药理活性,例如抗癌药[1],抗微生物药[2-4],抗氧化剂[5],抗增殖药[6],细胞毒活性[7],预期的抗肿瘤药[8],抗帕金森病[9]和抗炎[10]。此外,众所周知,吡喃衍生物可用于细菌生物膜破坏剂[11],抗惊厥药[12]和牛分枝杆菌的抑制剂[13]。
材料和方法:所有熔点均使用Akofler Block仪器测量,未经校正。红外光谱(KBr)记录在FTIR 5300光谱仪(υ,cm-1)上。1 H-NMR光谱在Varian Gemini光谱仪上记录。在作为溶剂的DMSO-d6,CDCl 3中,1 H-NMR谱在300、400 MHz下运行,13 C-NMR谱在100 MHz下运行。通过使用四甲基硅烷(TMS)作为内部参比,70 eV下的1000 EX质谱仪,化学位移以百万分之一(ppm)表示。通过薄层色谱法(TLC)(铝片)使用正己烷,EtOAc(9:1,V / V,7:3 V / V)洗脱液检查合成化合物的纯度。元素分析是由埃及开罗大学药理学院微分析研究中心和药学院微分析部门进行的。
结果与讨论:通过7-氨基-5-(4-氯苯基)-4-苯基-2-硫代-2-2,5-二氢-1H-吡喃基-[[ 2,3-d]嘧啶-6-腈1a和7-氨基-4,5-二苯基-2-thioxo-2,5-二氢-1H-吡喃并[2,3-d]-嘧啶-6-腈1b与一些亲电试剂和亲核试剂一起使用。通过元素分析和光谱数据确认了目标化合物的结构。新合成的化合物对前面提到的微生物显示出良好的抗菌活性。
结论:总之,化合物1a,1b已通过易于与不同试剂,在不同条件下反应而稠合的杂环化合物进行环化,并进行了抗菌筛选。











































 京公网安备 11010802027423号
京公网安备 11010802027423号