Current Organic Synthesis ( IF 1.7 ) Pub Date : 2020-10-31 , DOI: 10.2174/1570179417666200628015511 Ferkat Khaliullin 1 , Yuliya Shabalina 1
|
Aim and Objective: 1-Аlkyl-3,7-dihydro-1H-purine-2,6-diones containing no substituents in the N7 position can be synthesized only using protecting groups, for example, benzyl protection. However, in the case of synthesis of 1-benzyl-3,7-dihydro-1H-purine-2,6-diones, the use of benzyl protection may lead to simultaneous debenzylation of both N1 and N7 positions. Therefore, it is necessary to use other protective groups for the synthesis of 1-benzyl-3,7-dihydro-1H-purine-2,6-diones.
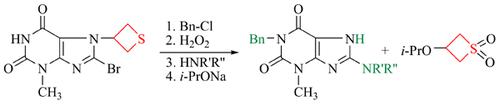
Materials and Methods: 8-Bromo- and 8-amino-substituted 1-benzyl-3-methyl-3,7-dihydro-1H-purine-2,6-diones unsubstituted in the N7 position were synthesized with the use of thietanyl protecting group. The thietane ring was introduced via the reaction of 8-bromo-3-methyl-3,7-dihydro-1H-purine-2,6-dione with 2-chloromethylthiirane, giving rise to 8-bromo-3-methyl-7-(thietan-3-yl)-3,7-dihydro-1H-purine-2,6-dione. The subsequent alkylation with benzyl chloride yielded 1-benzyl-8-bromo-3-methyl-7-(thietan-3-yl)-3,7-dihydro-1H-purine-2,6-dione, which was oxidized with hydrogen peroxide to be converted to 1-benzyl-8-bromo-3-methyl-7-(1,1-dioxothietan- 3-yl)-3,7-dihydro-1H-purine-2,6-dione. This product reacted with amines to give 8-amino-substituted 1-benzyl-3- methyl-7-(1,1-dioxothietan-3-yl)-3,7-dihydro-1H-purine-2,6-diones. The reaction of 8-substituted 1-benzyl-3- methyl-7-(1,1-dioxothietan-3-yl)-3,7-dihydro-1H-purine-2,6-diones with sodium isopropoxide resulted in the removal of the thietanyl protection and afforded target 8-substituted 1-benzyl-3-methyl-3,7-dihydro-1H-purine-2,6- diones. The structures of the targets compounds have been deduced upon their elemental analysis and spectral data (IR, 1H NMR, 13C NMR and 15N NMR).
Results and Discussion: A new 8-substituted 1-benzyl-3-methyl-3,7-dihydro-1H-purine-2,6-diones unsubstituted in the N7 position were synthesized using thietanyl protecting group.
Conclusion: The present study described a new route to synthesize some new 1,8-disubstituted 3-methyl-3,7- dihydro-1H-purine-2,6-diones unsubstituted in the N7 position starting from available 8-bromo-3-methyl-3,7- dihydro-1H-purine-2,6-dione with use of thietanyl protecting group. The advantages of this protocol are the possibility of the synthesis of 1-benzyl-substituted 3,7-dihydro-1H-purine-2,6-diones, the stability of the thietanyl protecting group upon nucleophilic substitution by amines of the bromine atom in the position 8, as well as mild conditions, and simple execution of experiments.
中文翻译:

合成8-取代的1-苄基-3-甲基-3,7-二氢-1H-嘌呤-2,6-二酮时的噻吩基保护。
目的和目的:仅在使用保护基(例如苄基保护)的情况下,才能合成在N7位不具有取代基的1-lkyl-3,7-二氢-1H-嘌呤-2,6-二酮。但是,在合成1-苄基-3,7-二氢-1H-嘌呤-2,6-二酮的情况下,苄基保护的使用可能导致N1和N7位同时脱苄基。因此,必须使用其他保护基来合成1-苄基-3,7-二氢-1H-嘌呤-2,6-二酮。
材料与方法:利用硫杂环丁烷基保护基合成了在N7位未取代的8-溴和8-氨基取代的1-苄基-3-甲基-3,7-二氢-1H-嘌呤-2,6-二酮组。通过8-溴-3-甲基-3,7-二氢-1H-嘌呤-2,6-二酮与2-氯甲基噻喃的反应引入硫杂环丁烷环,生成8-溴-3-甲基-7- (thietan-3-yl)-3,7-dihydro-1H-purine-2,6-dione。随后用苄基氯烷基化,得到1-苄基-8-溴-3-甲基-7-(噻吩-3-基)-3,7-二氢-1H-嘌呤-2,6-二酮,将其用氢气氧化过氧化物将其转化为1-苄基-8-溴-3-甲基-7-(1,1-二氧噻吩-3-基)-3,7-二氢-1H-嘌呤-2,6-二酮。该产物与胺反应,得到8-氨基取代的1-苄基-3-甲基-7-(1,1-二氧杂环丁烷-3-基)-3,7-二氢-1H-嘌呤-2,6-二酮。8-取代的1-苄基-3-甲基-7-(1,1-二氧噻吩-3-基)-3,7-二氢-1H-嘌呤-2,6-二酮与异丙醇的反应可除去的噻吩基保护基,得到目标的8-取代的1-苄基-3-甲基-3,7-二氢-1H-嘌呤-2,6-二酮。根据目标化合物的元素分析和光谱数据(IR,1H NMR,13C NMR和15N NMR)推导了目标化合物的结构。
结果与讨论:利用硫杂环丁烷基保护基合成了在N7位未被取代的8-取代的1-苄基-3-甲基-3,7-二氢-1H-嘌呤-2,6-二酮。
结论:本研究描述了一条新的路线,可以合成一些新的从8-溴-3开始的,在N7位置未被取代的1,8-二取代的3-甲基-3,7-二氢-1H-嘌呤-2,6-二酮。 -具有噻吩基保护基的-甲基-3,7-二氢-1H-嘌呤-2,6-二酮。该方案的优点是可以合成1-苄基取代的3,7-二氢-1H-嘌呤-2,6-二酮,噻吩基保护基在亲核被溴原子的胺取代后的稳定性。位置8,以及温和的条件和简单的实验执行。











































 京公网安备 11010802027423号
京公网安备 11010802027423号