当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Deconjugated butenolide: a versatile building block for asymmetric catalysis.
Chemical Society Reviews ( IF 46.2 ) Pub Date : 2020-08-12 , DOI: 10.1039/c9cs00346k Abhijnan Ray Choudhury 1 , Santanu Mukherjee 1
Chemical Society Reviews ( IF 46.2 ) Pub Date : 2020-08-12 , DOI: 10.1039/c9cs00346k Abhijnan Ray Choudhury 1 , Santanu Mukherjee 1
Affiliation
|
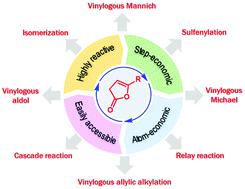
The wide abundance of γ-lactones in natural products and bioactive targets calls for suitable building blocks for their enantioselective synthesis. β,γ-Unsaturated γ-butenolides, commonly known as deconjugated butenolides, owing to their easy accessibility and highly reactive nature, have emerged as the synthon of choice during the past decade for the enantioselective synthesis of γ-lactones. Their compatibility under organocatalytic, metal-catalyzed as well as cooperative catalytic conditions has resulted in numerous enantioselective transformations involving deconjugated butenolides. These reactions not only led to enantioenriched γ-lactones, but also various other heterocycles and acyclic compounds through ring-opening and fragmentation of the parent butenolide ring. The purpose of this review is to provide a comprehensive treatise on the catalytic asymmetric reactions of deconjugated butenolides reported so far. This aspect is presented alongside the preparation and reactivity comparison of deconjugated butenolides with other competing synthons of γ-lactones. Limitations of the existing protocols and the possible scope for future development are also discussed.
中文翻译:

去共轭丁烯内酯:一种用于不对称催化的通用结构单元。
天然产物和具有生物活性的靶标中存在大量的γ-内酯,因此需要合适的结构单元来进行对映体选择性合成。β,γ-不饱和γ-丁烯化物,由于易于获取和高反应性,通常被称为共轭丁烯化物,在过去的十年中已成为γ-内酯对映选择性合成的首选合成子。它们在有机催化,金属催化以及协同催化条件下的相容性已导致涉及对位丁烯内酯的许多对映选择性转化。这些反应不仅导致对映体富集的γ-内酯,而且通过开环和母体丁烯内酯环的断裂而导致各种其他杂环和无环化合物。这篇综述的目的是提供迄今为止所报道的去结合的丁烯内酯的催化不对称反应的全面论述。在这方面与解偶联的丁烯内酯与γ-内酯的其他竞争性合成子的制备和反应性比较一起提出。还讨论了现有协议的局限性以及未来开发的可能范围。
更新日期:2020-09-21
中文翻译:

去共轭丁烯内酯:一种用于不对称催化的通用结构单元。
天然产物和具有生物活性的靶标中存在大量的γ-内酯,因此需要合适的结构单元来进行对映体选择性合成。β,γ-不饱和γ-丁烯化物,由于易于获取和高反应性,通常被称为共轭丁烯化物,在过去的十年中已成为γ-内酯对映选择性合成的首选合成子。它们在有机催化,金属催化以及协同催化条件下的相容性已导致涉及对位丁烯内酯的许多对映选择性转化。这些反应不仅导致对映体富集的γ-内酯,而且通过开环和母体丁烯内酯环的断裂而导致各种其他杂环和无环化合物。这篇综述的目的是提供迄今为止所报道的去结合的丁烯内酯的催化不对称反应的全面论述。在这方面与解偶联的丁烯内酯与γ-内酯的其他竞争性合成子的制备和反应性比较一起提出。还讨论了现有协议的局限性以及未来开发的可能范围。



























 京公网安备 11010802027423号
京公网安备 11010802027423号