当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nanoscale Relaxation in "Water-in-Salt" and "Water-in-Bisalt" Electrolytes.
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2020-08-12 , DOI: 10.1021/acs.jpclett.0c01765 Miguel A González 1 , Oleg Borodin 2 , Maiko Kofu 3 , Kaoru Shibata 3 , Takeshi Yamada 4 , Osamu Yamamuro 5 , Kang Xu 2 , David L Price 6 , Marie-Louise Saboungi 7
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2020-08-12 , DOI: 10.1021/acs.jpclett.0c01765 Miguel A González 1 , Oleg Borodin 2 , Maiko Kofu 3 , Kaoru Shibata 3 , Takeshi Yamada 4 , Osamu Yamamuro 5 , Kang Xu 2 , David L Price 6 , Marie-Louise Saboungi 7
Affiliation
|
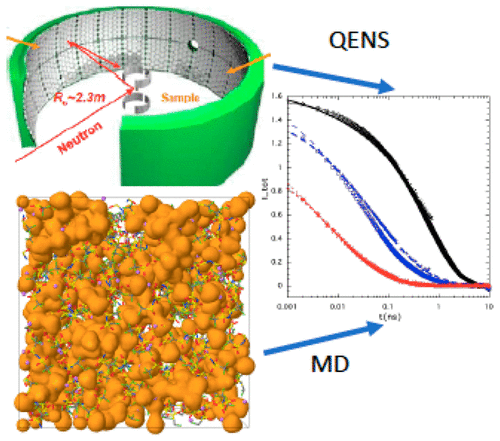
“Water-in-salt” (WIS) and “water-in-bisalt” (WIBS) electrolytes have recently been developed for Li-ion batteries, combining the safety and environmental friendliness of aqueous electrolytes with a larger operating window made possible by a solid-electrolyte interphase. We report quasielastic neutron scattering (QENS) measurements on solutions of a WIS electrolyte at two concentrations, 13.9 and 21 m (molal) lithium bis(trifluoromethane)sulfonimide LiTFSI in H2O/D2O and a WIBS electrolyte at (21 m LiTFSI + 7 m lithium triflate (LiOTf)) in H2O/D2O. The data were Fourier transformed to obtain experimental intermediate scattering functions (ISFs) and compared with corresponding quantities obtained from molecular dynamics (MD) simulations. Both QENS and MD ISFs could be fitted well by a single stretched exponential function to obtain apparent translational diffusion coefficients for the water molecules. The QENS values agree well with the MD simulations for the 13.9 and 21 m solutions, but MD simulations predict a slower relaxation of water compared to QENS for the WIBS electrolyte. Comparison of the incoherent and coherent scattering reveals much faster water dynamics compared with structural relaxation of the ionic framework, consistent with the nanodomain picture where the lithium diffusion occurs through the tortuous water domain around the slower relaxing ionic matrix, leading to highly non-Gaussian water motion.
中文翻译:

“盐包水”和“盐析水”电解质中的纳米尺度弛豫。
最近已为锂离子电池开发了“盐包水”(WIS)和“双盐包水”(WIBS)电解质,将水性电解质的安全性和环境友好性与更大的操作范围相结合固体电解质界面。我们报告了在两种浓度的WIS电解质溶液中的准弹性中子散射(QENS)测量,两种浓度分别是H 2 O / D 2 O中的13.9和21 m(摩尔)双(三氟甲烷)磺酰亚胺锂LiTFSI和(21 m LiTFSI)WIBS电解质+ 7 m三氟甲磺酸锂(LiOTf))在H 2 O / D 2中O.对数据进行傅立叶变换以获得实验中间散射函数(ISF),并将其与从分子动力学(MD)模拟获得的相应数量进行比较。QENS和MD ISF都可以通过单个拉伸指数函数很好地拟合,以获得水分子的表观平移扩散系数。QENS值与13.9和21 m的MD模拟非常吻合解决方案,但MD模拟预测,与WIBS电解质的QENS相比,水的弛豫速度更慢。非相干和相干散射的比较显示,与离子骨架的结构弛豫相比,水动力学要快得多,这与纳米域图一致,其中锂扩散通过较慢弛豫离子基质周围的曲折水域发生,从而导致高度非高斯水运动。
更新日期:2020-09-03
中文翻译:

“盐包水”和“盐析水”电解质中的纳米尺度弛豫。
最近已为锂离子电池开发了“盐包水”(WIS)和“双盐包水”(WIBS)电解质,将水性电解质的安全性和环境友好性与更大的操作范围相结合固体电解质界面。我们报告了在两种浓度的WIS电解质溶液中的准弹性中子散射(QENS)测量,两种浓度分别是H 2 O / D 2 O中的13.9和21 m(摩尔)双(三氟甲烷)磺酰亚胺锂LiTFSI和(21 m LiTFSI)WIBS电解质+ 7 m三氟甲磺酸锂(LiOTf))在H 2 O / D 2中O.对数据进行傅立叶变换以获得实验中间散射函数(ISF),并将其与从分子动力学(MD)模拟获得的相应数量进行比较。QENS和MD ISF都可以通过单个拉伸指数函数很好地拟合,以获得水分子的表观平移扩散系数。QENS值与13.9和21 m的MD模拟非常吻合解决方案,但MD模拟预测,与WIBS电解质的QENS相比,水的弛豫速度更慢。非相干和相干散射的比较显示,与离子骨架的结构弛豫相比,水动力学要快得多,这与纳米域图一致,其中锂扩散通过较慢弛豫离子基质周围的曲折水域发生,从而导致高度非高斯水运动。

































 京公网安备 11010802027423号
京公网安备 11010802027423号