当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Macroscopic Differentiators for Microscopic Structural Nonideality in Binary Ionic Liquid Mixtures.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-08-11 , DOI: 10.1021/acs.jpcb.0c03740 Utkarsh Kapoor 1 , Jindal K Shah 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-08-11 , DOI: 10.1021/acs.jpcb.0c03740 Utkarsh Kapoor 1 , Jindal K Shah 1
Affiliation
|
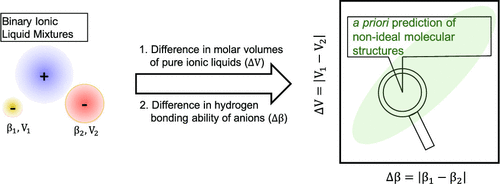
Combining two ionic liquids to form a binary ionic liquid mixture is a simple yet effective strategy to not only expand the number of ionic liquids but also precisely control various physicochemical properties of resultant ionic liquid mixtures. From a fundamental thermodynamic point of view, it is not entirely clear whether such mixtures can be classified as ideal solutions. Given a large number of binary ionic liquid mixtures that emerge, the ability to predict the presence of nonideality in such mixtures a priori without the need for experimentation or molecular simulation-based calculations is immensely valuable for their rational design. In this research report, we demonstrate that the difference in the molar volumes (ΔV) of the pure ionic liquids and the difference in the hydrogen-bonding ability of anions (Δβ) are the primary determinants of nonideal behavior of binary ionic liquid mixtures containing a common cation and two anions. Our conclusion is derived from a comparison of microscopic structural properties expressed in terms of radial, spatial, and angular distributions for binary mixtures and those of the corresponding pure ionic liquids. Molecular dynamics simulations of 16 binary ionic liquid mixtures, containing a common cation 1-n-butyl-3-methylimidazolium [C4mim]+ and combinations of (less basic) fluorinated {trifluoromethylacetate [TFA]−, trifluoromethanesulfonate [TFS]−, bis(trifluoromethanesulfonyl)imide [NTf2]−, and tris (pentafluoroethyl) trifluorophosphate [eFAP]−} versus (more basic) nonfluorinated {chloride Cl–, acetate [OAC]−, methylsulfate [MeSO4]−, and dimethylphosphate [Me2PO4]−} anions, were conducted. The large number of binary ionic liquid mixtures examined here enabled us to span a broad range of ΔV and Δβ values. The results indicate that binary mixtures of two ionic liquids for which ΔV > 60 cm3/mol and Δβ > 0.4 are expected to be microscopically nonideal. On the other hand, ΔV < 60 cm3/mol and Δβ < 0.4 will lead to molecular structures that are not differentiated from those of their pure ionic liquid counterparts.
中文翻译:

二元离子液体混合物中微观结构非理想的宏观微分器。
结合两种离子液体以形成二元离子液体混合物是一种简单而有效的策略,不仅可以扩大离子液体的数量,而且可以精确控制所得离子液体混合物的各种物理化学性质。从基本的热力学观点来看,尚不清楚这种混合物是否可以归类为理想溶液。鉴于出现了大量的二元离子液体混合物,无需进行实验或基于分子模拟的计算就可以事先预测此类混合物中非理想成分的存在,对其合理设计具有巨大的价值。在本研究报告中,我们证明了摩尔体积的差异(ΔV离子和两种阴离子的二元离子液体混合物的非理想行为的主要决定因素是纯离子液体的)和阴离子氢键能力的差异(Δβ)。我们的结论源自对二元混合物和相应的纯离子液体的微观结构特性的径向,空间和角度分布表示的比较。16种二元离子液体混合物的分子动力学模拟,这些混合物包含普通阳离子1-正丁基-3-甲基咪唑鎓[C 4 mim] +和(弱碱性)氟化三氟甲基乙酸盐[TFA] -,三氟甲磺酸盐[TFS] -的组合,双(三氟甲烷磺酰)亚胺[NTF 2 ] - ,和三(五氟乙基)三氟[EFAP] - }与(更碱性)非氟化{氯化氯- ,乙酸酯[OAC] - ,甲基硫酸[内消旋4 ] - ,和二甲基磷酸[进行了Me 2 PO 4 ] - }阴离子。此处检查的大量二元离子液体混合物使我们能够跨越很宽的ΔV和Δβ值范围。结果表明,ΔV > 60 cm 3的两种离子液体的二元混合物/ mol和Δβ> 0.4预计在显微镜下是不理想的。另一方面,ΔV <60 cm 3 / mol和Δβ<0.4将导致分子结构与其纯离子液体对应物没有区别。
更新日期:2020-09-10
中文翻译:

二元离子液体混合物中微观结构非理想的宏观微分器。
结合两种离子液体以形成二元离子液体混合物是一种简单而有效的策略,不仅可以扩大离子液体的数量,而且可以精确控制所得离子液体混合物的各种物理化学性质。从基本的热力学观点来看,尚不清楚这种混合物是否可以归类为理想溶液。鉴于出现了大量的二元离子液体混合物,无需进行实验或基于分子模拟的计算就可以事先预测此类混合物中非理想成分的存在,对其合理设计具有巨大的价值。在本研究报告中,我们证明了摩尔体积的差异(ΔV离子和两种阴离子的二元离子液体混合物的非理想行为的主要决定因素是纯离子液体的)和阴离子氢键能力的差异(Δβ)。我们的结论源自对二元混合物和相应的纯离子液体的微观结构特性的径向,空间和角度分布表示的比较。16种二元离子液体混合物的分子动力学模拟,这些混合物包含普通阳离子1-正丁基-3-甲基咪唑鎓[C 4 mim] +和(弱碱性)氟化三氟甲基乙酸盐[TFA] -,三氟甲磺酸盐[TFS] -的组合,双(三氟甲烷磺酰)亚胺[NTF 2 ] - ,和三(五氟乙基)三氟[EFAP] - }与(更碱性)非氟化{氯化氯- ,乙酸酯[OAC] - ,甲基硫酸[内消旋4 ] - ,和二甲基磷酸[进行了Me 2 PO 4 ] - }阴离子。此处检查的大量二元离子液体混合物使我们能够跨越很宽的ΔV和Δβ值范围。结果表明,ΔV > 60 cm 3的两种离子液体的二元混合物/ mol和Δβ> 0.4预计在显微镜下是不理想的。另一方面,ΔV <60 cm 3 / mol和Δβ<0.4将导致分子结构与其纯离子液体对应物没有区别。











































 京公网安备 11010802027423号
京公网安备 11010802027423号