当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodium(III)-Catalyzed Oxidative ortho-Olefination of Phenyl Carbamates with Alkenes: Elucidation of Acceleration Mechanisms by Using an Unsubstituted Cyclopentadienyl Ligand.
Organic Letters ( IF 4.9 ) Pub Date : 2020-08-12 , DOI: 10.1021/acs.orglett.0c02499 Jin Tanaka 1 , Yuki Nagashima 1 , Ken Tanaka 1
Organic Letters ( IF 4.9 ) Pub Date : 2020-08-12 , DOI: 10.1021/acs.orglett.0c02499 Jin Tanaka 1 , Yuki Nagashima 1 , Ken Tanaka 1
Affiliation
|
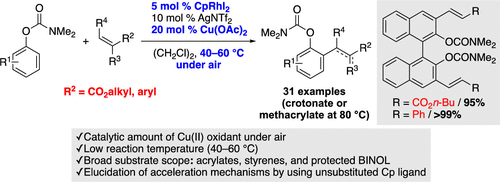
It has been established that an unsubstituted cyclopentadienyl (Cp) Rh(III) complex is an effective catalyst for the oxidative ortho-olefination of phenyl carbamates with both acrylates and styrenes under mild conditions. In addition, diolefination of a protected BINOL (1,1′-binaphthalene-2,2′-diol) proceeded in high yields and disubstituted acrylates could participate in this catalysis. Experimental and theoretical mechanistic studies elucidated that an electron-deficient nature of the unsubstituted CpRh(III) complex accelerates both the electrophilic aryl C–H rhodation and the rate-limiting alkene insertion steps.
中文翻译:

铑(III)催化的烯基苯甲酸氨基甲酸酯的氧化邻位加氢精制:通过使用未取代的环戊二烯基配体阐明加速机理。
已经确定,在温和条件下,未取代的环戊二烯基(Cp)Rh(III)配合物是苯基氨基甲酸酯与丙烯酸酯和苯乙烯的氧化邻位烯烃聚合的有效催化剂。此外,受保护的BINOL(1,1'-联萘-2,2'-二醇)的二烯化反应产率很高,二取代的丙烯酸酯可参与该催化作用。实验和理论机理研究表明,未取代的CpRh(III)络合物的电子缺陷性质会加速亲电芳基CH的铑化反应和限速烯烃的插入步骤。
更新日期:2020-09-20
中文翻译:

铑(III)催化的烯基苯甲酸氨基甲酸酯的氧化邻位加氢精制:通过使用未取代的环戊二烯基配体阐明加速机理。
已经确定,在温和条件下,未取代的环戊二烯基(Cp)Rh(III)配合物是苯基氨基甲酸酯与丙烯酸酯和苯乙烯的氧化邻位烯烃聚合的有效催化剂。此外,受保护的BINOL(1,1'-联萘-2,2'-二醇)的二烯化反应产率很高,二取代的丙烯酸酯可参与该催化作用。实验和理论机理研究表明,未取代的CpRh(III)络合物的电子缺陷性质会加速亲电芳基CH的铑化反应和限速烯烃的插入步骤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号