当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Enantioselective Synthesis and Switchable Chiroptical Property of Inherently Chiral Macrocycles
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-08-12 , DOI: 10.1021/jacs.0c05369 Shuo Tong 1 , Jiang-Tao Li 1 , Dong-Dong Liang 1 , Yan-E Zhang 1 , Qi-Yun Feng 1 , Xin Zhang 1 , Jieping Zhu 2 , Mei-Xiang Wang 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-08-12 , DOI: 10.1021/jacs.0c05369 Shuo Tong 1 , Jiang-Tao Li 1 , Dong-Dong Liang 1 , Yan-E Zhang 1 , Qi-Yun Feng 1 , Xin Zhang 1 , Jieping Zhu 2 , Mei-Xiang Wang 1
Affiliation
|
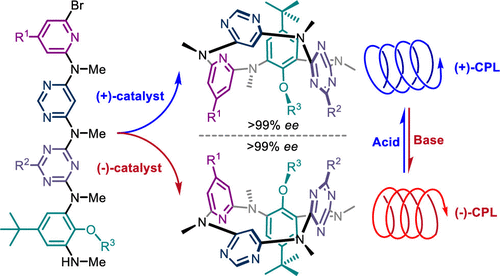
We report herein a strategy to construct enantiopure inherently chiral macrocycles, ABCD-type heteracalix[4]aromatics, through a catalytic enantioselective intramolecular C-N bond forming reaction. A chiral ligand-palladium complex was found to efficiently induce the inherent chirality of molecules during macrocyclization process with ee values up to >99%. The resulting ABCD-type heteracalix[4]aromatics displayed excellent and pH-triggered switchable electronic circular dichroism and circularly polarized luminescence properties.
中文翻译:

固有手性大环化合物的催化对映选择性合成和可切换的手性
我们在此报告了一种通过催化对映选择性分子内 CN 键形成反应构建对映纯的固有手性大环、ABCD 型杂杯 [4] 芳烃的策略。发现手性配体-钯配合物在大环化过程中有效地诱导分子的固有手性,ee 值高达 >99%。由此产生的 ABCD 型杂杯 [4] 芳烃显示出优异的和 pH 触发的可切换电子圆二色性和圆偏振发光特性。
更新日期:2020-08-12
中文翻译:

固有手性大环化合物的催化对映选择性合成和可切换的手性
我们在此报告了一种通过催化对映选择性分子内 CN 键形成反应构建对映纯的固有手性大环、ABCD 型杂杯 [4] 芳烃的策略。发现手性配体-钯配合物在大环化过程中有效地诱导分子的固有手性,ee 值高达 >99%。由此产生的 ABCD 型杂杯 [4] 芳烃显示出优异的和 pH 触发的可切换电子圆二色性和圆偏振发光特性。










































 京公网安备 11010802027423号
京公网安备 11010802027423号