当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structure and Function of NzeB, a Versatile C–C and C–N Bond Forming Diketopiperazine Dimerase
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-08-12 , DOI: 10.1021/jacs.0c06312 Vikram V Shende , Yogan Khatri , Sean A Newmister , Jacob N Sanders 1 , Petra Lindovska 2 , Fengan Yu , Tyler J Doyon , Justin Kim 2 , K N Houk 1 , Mohammad Movassaghi 2 , David H Sherman
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-08-12 , DOI: 10.1021/jacs.0c06312 Vikram V Shende , Yogan Khatri , Sean A Newmister , Jacob N Sanders 1 , Petra Lindovska 2 , Fengan Yu , Tyler J Doyon , Justin Kim 2 , K N Houk 1 , Mohammad Movassaghi 2 , David H Sherman
Affiliation
|
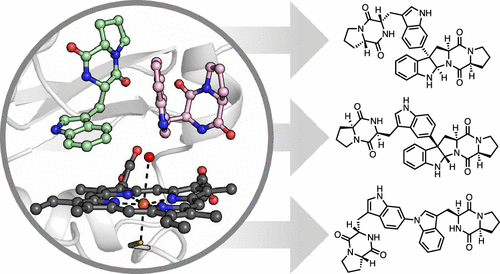
The dimeric diketopiperazine (DKPs) alkaloids are a diverse family of natural products (NPs) whose unique structural architectures and biological activities have inspired the development of new synthetic methodology to access these molecules. However, catalyst-controlled methods that enable the selective formation of constitutional and stereoisomeric dimers from a single monomer are lacking. To resolve this long-standing synthetic challenge, we sought to characterize the biosynthetic enzymes that assemble these NPs for application in biocatalytic syntheses. Genome mining enabled identification of the cytochrome P450, NzeB (derived from Streptomyces sp. NRRL F-5053), which catalyzes both intermolecular carbon-carbon (C-C) and carbon-nitrogen (C-N) bond formation, generating all currently known DKP dimer scaffolds isolated from bacterial sources. To identify the molecular basis for the flexible site-, stereo-, and chemoselectivity of NzeB, we obtained high-resolution crystal structures (1.5Å) of the protein in complex with native and non-native substrates. This, to our knowledge, represents the first crystal structure of an oxidase catalyzing direct, intermolecular C-H amination. Site-directed mutagenesis was employed to assess the role individual active site residues play in guiding selective DKP dimerization. Finally, computational approaches were employed to evaluate plausible mechanisms regarding NzeB function and its ability to catalyze both C-C and C-N bond formation. These results provide a structural and computational rationale for the catalytic versatility of NzeB, as well as new insights into variables that control selectivity of CYP450 diketopiperazine dimerases.
中文翻译:

NzeB 的结构和功能,一种多功能的 C-C 和 C-N 键形成二酮哌嗪二聚酶
二聚二酮哌嗪 (DKPs) 生物碱是一个多样化的天然产物 (NPs) 家族,其独特的结构结构和生物活性激发了开发新的合成方法来获取这些分子。然而,缺乏能够从单个单体选择性形成结构和立体异构二聚体的催化剂控制方法。为了解决这一长期存在的合成挑战,我们试图表征组装这些 NPs 以用于生物催化合成的生物合成酶。基因组挖掘能够鉴定细胞色素 P450、NzeB(源自 Streptomyces sp. NRRL F-5053),它催化分子间碳 - 碳(CC)和碳 - 氮(CN)键的形成,生成所有目前已知的分离的 DKP 二聚体支架从细菌来源。为了确定 NzeB 灵活的位点、立体和化学选择性的分子基础,我们获得了与天然和非天然底物复合的蛋白质的高分辨率晶体结构 (1.5Å)。据我们所知,这代表了催化直接分子间 CH 胺化的氧化酶的第一个晶体结构。采用定点诱变来评估单个活性位点残基在指导选择性 DKP 二聚化中的作用。最后,采用计算方法来评估关于 NzeB 功能的合理机制及其催化 CC 和 CN 键形成的能力。这些结果为 NzeB 的催化多功能性提供了结构和计算原理,以及对控制 CYP450 二酮哌嗪二聚酶选择性的变量的新见解。
更新日期:2020-08-12
中文翻译:

NzeB 的结构和功能,一种多功能的 C-C 和 C-N 键形成二酮哌嗪二聚酶
二聚二酮哌嗪 (DKPs) 生物碱是一个多样化的天然产物 (NPs) 家族,其独特的结构结构和生物活性激发了开发新的合成方法来获取这些分子。然而,缺乏能够从单个单体选择性形成结构和立体异构二聚体的催化剂控制方法。为了解决这一长期存在的合成挑战,我们试图表征组装这些 NPs 以用于生物催化合成的生物合成酶。基因组挖掘能够鉴定细胞色素 P450、NzeB(源自 Streptomyces sp. NRRL F-5053),它催化分子间碳 - 碳(CC)和碳 - 氮(CN)键的形成,生成所有目前已知的分离的 DKP 二聚体支架从细菌来源。为了确定 NzeB 灵活的位点、立体和化学选择性的分子基础,我们获得了与天然和非天然底物复合的蛋白质的高分辨率晶体结构 (1.5Å)。据我们所知,这代表了催化直接分子间 CH 胺化的氧化酶的第一个晶体结构。采用定点诱变来评估单个活性位点残基在指导选择性 DKP 二聚化中的作用。最后,采用计算方法来评估关于 NzeB 功能的合理机制及其催化 CC 和 CN 键形成的能力。这些结果为 NzeB 的催化多功能性提供了结构和计算原理,以及对控制 CYP450 二酮哌嗪二聚酶选择性的变量的新见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号