当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced Redox Reactivity of a Nonheme Iron(V)‒Oxo Complex Binding Protons
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-08-12 , DOI: 10.1021/jacs.0c05108 Shan-Shan Xue 1 , Xiao-Xi Li 1 , Yong-Min Lee 1 , Mi Sook Seo 1 , Yujeong Kim 1, 2 , Sachiko Yanagisawa 3 , Minoru Kubo 3 , Young-Kyo Jeon 1 , Won-Suk Kim 1 , Ritimukta Sarangi 4 , Sun Hee Kim 1, 2 , Shunichi Fukuzumi 1 , Wonwoo Nam 1, 5
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-08-12 , DOI: 10.1021/jacs.0c05108 Shan-Shan Xue 1 , Xiao-Xi Li 1 , Yong-Min Lee 1 , Mi Sook Seo 1 , Yujeong Kim 1, 2 , Sachiko Yanagisawa 3 , Minoru Kubo 3 , Young-Kyo Jeon 1 , Won-Suk Kim 1 , Ritimukta Sarangi 4 , Sun Hee Kim 1, 2 , Shunichi Fukuzumi 1 , Wonwoo Nam 1, 5
Affiliation
|
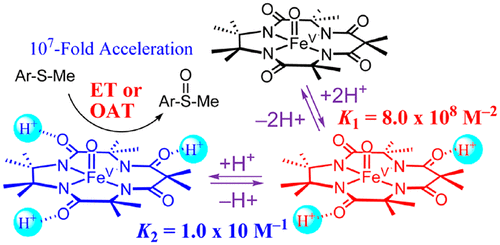
Acid effects on the chemical properties of metal-oxygen intermediates have attracted much attention recently, such as the enhanced reactivity of high-valent metal(IV)‒oxo species by binding proton(s) or Lewis acidic metal ion(s) in redox reactions. Herein, we report for the first time the proton effects of an iron(V)‒oxo complex bearing a negatively charged tetraamido macrocyclic ligand (TAML) in oxygen atom transfer (OAT) and electron-transfer (ET) reactions. First, we synthesized and characterized a mononuclear nonheme Fe(V)‒oxo TAML complex (1) and its protonated iron(V)‒oxo complexes binding two and three protons, which are denoted as 2 and 3, respectively. The protons were found to bind to the TAML ligand of the Fe(V)‒oxo species based on spectroscopic characterization, such as resonance Raman, EXAFS, and EPR measurements, along with DFT calculations. Two protons-binding constant of 1 to produce 2 and the third protonation constant of 2 to produce 3 were determined to be 8.0(7) × 108 M-2 and 10(1) M-1, respectively. The reactivities of the proton-bound iron(V)‒oxo complexes were investigated in OAT and ET reactions, showing a dramatic increase in the rate of sulfoxidation of thioanisole derivatives, such as 107 times increase in reactivity when the oxidation of p-CN-thioanisole by 1 was performed in the presence of HOTf (i.e., 200 mM). The one-electron reduction potential of 2 (Ered vs SCE = 0.97 V) was significantly shifted to the positive direction, compared to that of 1 (Ered vs SCE = 0.33 V). Upon further addition of proton to a solution of 2, more positive shift of Ered value was observed with a slope of 47 mV/log([HOTf]). The sulfoxidation of thioanisole derivatives by 2 was shown to proceed via ET from thioanisoles to 2 or direct OAT from 2 to thioanisoles, depending on the ET driving force.
中文翻译:

非血红素铁(V)-氧杂结合质子的氧化还原反应性增强
酸对金属氧中间体化学性质的影响最近引起了很多关注,例如通过在氧化还原反应中结合质子或路易斯酸性金属离子来增强高价金属(IV)-羰基化合物的反应性. 在此,我们首次报道了带有带负电荷的四酰氨基大环配体 (TAML) 的铁 (V)-氧配合物在氧原子转移 (OAT) 和电子转移 (ET) 反应中的质子效应。首先,我们合成并表征了单核非血红素 Fe(V)-oxo TAML 复合物 (1) 及其质子化铁 (V)-oxo 复合物,结合两个和三个质子,分别表示为 2 和 3。基于光谱表征,如共振拉曼、EXAFS 和 EPR 测量,发现质子与 Fe(V)-oxo 物种的 TAML 配体结合,以及 DFT 计算。两个质子结合常数 1 产生 2 和第三个质子结合常数 2 产生 3 分别确定为 8.0(7) × 108 M-2 和 10(1) M-1。在 OAT 和 ET 反应中研究了质子结合的铁 (V)-氧配合物的反应性,表明苯硫醚衍生物的磺化速率显着增加,例如当 p-CN-氧化时反应性增加 107 倍在 HOTf (即 200 mM) 存在下进行 1 次茴香硫醚的分析。与 1 (Ered vs SCE = 0.33 V) 相比,2 (Ered vs SCE = 0.97 V) 的单电子还原电位显着向正方向移动。在进一步向 2 的溶液中加入质子后,观察到 Ered 值的更多正偏移,斜率为 47 mV/log([HOTf])。
更新日期:2020-08-12
中文翻译:

非血红素铁(V)-氧杂结合质子的氧化还原反应性增强
酸对金属氧中间体化学性质的影响最近引起了很多关注,例如通过在氧化还原反应中结合质子或路易斯酸性金属离子来增强高价金属(IV)-羰基化合物的反应性. 在此,我们首次报道了带有带负电荷的四酰氨基大环配体 (TAML) 的铁 (V)-氧配合物在氧原子转移 (OAT) 和电子转移 (ET) 反应中的质子效应。首先,我们合成并表征了单核非血红素 Fe(V)-oxo TAML 复合物 (1) 及其质子化铁 (V)-oxo 复合物,结合两个和三个质子,分别表示为 2 和 3。基于光谱表征,如共振拉曼、EXAFS 和 EPR 测量,发现质子与 Fe(V)-oxo 物种的 TAML 配体结合,以及 DFT 计算。两个质子结合常数 1 产生 2 和第三个质子结合常数 2 产生 3 分别确定为 8.0(7) × 108 M-2 和 10(1) M-1。在 OAT 和 ET 反应中研究了质子结合的铁 (V)-氧配合物的反应性,表明苯硫醚衍生物的磺化速率显着增加,例如当 p-CN-氧化时反应性增加 107 倍在 HOTf (即 200 mM) 存在下进行 1 次茴香硫醚的分析。与 1 (Ered vs SCE = 0.33 V) 相比,2 (Ered vs SCE = 0.97 V) 的单电子还原电位显着向正方向移动。在进一步向 2 的溶液中加入质子后,观察到 Ered 值的更多正偏移,斜率为 47 mV/log([HOTf])。











































 京公网安备 11010802027423号
京公网安备 11010802027423号