当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chemical Probes for Blocking of Influenza A M2 Wild-type and S31N Channels.
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-08-12 , DOI: 10.1021/acschembio.0c00553 Christina Tzitzoglaki 1 , Kelly McGuire 2 , Panagiotis Lagarias 1 , Athina Konstantinidi 1 , Anja Hoffmann 3 , Natalie A Fokina 4 , Chulong Ma 5 , Ioannis P Papanastasiou 1 , Peter R Schreiner 4 , Santiago Vázquez 6 , Michaela Schmidtke 3 , Jun Wang 5 , David D Busath 2 , Antonios Kolocouris 1
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-08-12 , DOI: 10.1021/acschembio.0c00553 Christina Tzitzoglaki 1 , Kelly McGuire 2 , Panagiotis Lagarias 1 , Athina Konstantinidi 1 , Anja Hoffmann 3 , Natalie A Fokina 4 , Chulong Ma 5 , Ioannis P Papanastasiou 1 , Peter R Schreiner 4 , Santiago Vázquez 6 , Michaela Schmidtke 3 , Jun Wang 5 , David D Busath 2 , Antonios Kolocouris 1
Affiliation
|
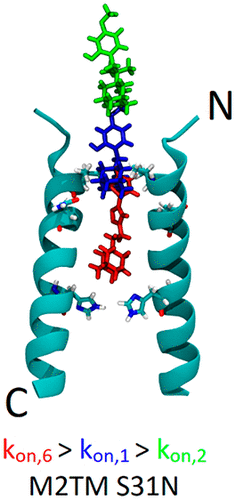
We report on using the synthetic aminoadamantane-CH2-aryl derivatives 1–6 as sensitive probes for blocking M2 S31N and influenza A virus (IAV) M2 wild-type (WT) channels as well as virus replication in cell culture. The binding kinetics measured using electrophysiology (EP) for M2 S31N channel are very dependent on the length between the adamantane moiety and the first ring of the aryl headgroup realized in 2 and 3 and the girth and length of the adamantane adduct realized in 4 and 5. Study of 1–6 shows that, according to molecular dynamics (MD) simulations and molecular mechanics Poisson–Boltzmann surface area (MM/PBSA) calculations, all bind in the M2 S31N channel with the adamantyl group positioned between V27 and G34 and the aryl group projecting out of the channel with the phenyl (or isoxazole in 6) embedded in the V27 cluster. In this outward binding configuration, an elongation of the ligand by only one methylene in rimantadine 2 or using diamantane or triamantane instead of adamantane in 4 and 5, respectively, causes incomplete entry and facilitates exit, abolishing effective block compared to the amantadine derivatives 1 and 6. In the active M2 S31N blockers 1 and 6, the phenyl and isoxazolyl head groups achieve a deeper binding position and high kon/low koff and high kon/high koff rate constants, compared to inactive 2–5, which have much lower kon and higher koff. Compounds 1–5 block the M2 WT channel by binding in the longer area from V27–H37, in the inward orientation, with high kon and low koff rate constants. Infection of cell cultures by influenza virus containing M2 WT or M2 S31N is inhibited by 1–5 or 1–4 and 6, respectively. While 1 and 6 block infection through the M2 block mechanism in the S31N variant, 2–4 may block M2 S31N virus replication in cell culture through the lysosomotropic effect, just as chloroquine is thought to inhibit SARS-CoV-2 infection.
中文翻译:

用于阻断A型M2流感野生型和S31N通道的化学探针。
我们使用合成的氨基金刚烷-CH报告2 -芳基衍生物1 - 6作为用于阻断M2 S31N和A型流感病毒(IAV)M2的野生型(WT)信道以及在细胞培养中的病毒复制敏感探针。使用电生理学(EP)测量的M2 S31N通道的结合动力学非常依赖于在2和3中实现的金刚烷部分与芳基首基的第一环之间的长度以及在4和5中实现的金刚烷加合物的周长和长度。研究1 – 6表明,根据分子动力学(MD)模拟和分子力学泊松-玻尔兹曼表面积(MM / PBSA)计算,所有分子均在M2 S31N通道中结合,金刚烷基位于V27和G34之间,而芳基则突出于苯基(或6中的异恶唑)嵌入V27簇中的通道。在该向外结合构型中,金刚烷胺2中仅一种亚甲基的配体伸长或分别在4和5中使用金刚烷或金刚烷而不是金刚烷的配体伸长导致不完全进入并促进退出,与金刚烷胺衍生物1和2相比,消除了有效的嵌段。6。在活动的M2 S31N阻止器中1和6,苯基和异恶唑基头部基团实现更深的装订位置和高ķ上/低ķ关闭和高ķ上/高ķ离速率常数相比,不活动的2 - 5,其具有低得多的ķ上和更高ķ关。化合物1 - 5块由在从V27-H37的较长区域结合,在向内取向M2 WT信道,具有高ķ上和低ķ关闭速率常数。通过含有M2 WT或M2 S31N流感病毒的细胞培养物的感染是通过抑制1 - 5或1 - 4和6分别。而1和6块感染通过在S31N的变体M2块机构,2 - 4可通过溶酶体效应阻断在细胞培养物M2 S31N病毒复制,正如氯喹被认为能够抑制SARS-CoV的-2感染。
更新日期:2020-09-20
中文翻译:

用于阻断A型M2流感野生型和S31N通道的化学探针。
我们使用合成的氨基金刚烷-CH报告2 -芳基衍生物1 - 6作为用于阻断M2 S31N和A型流感病毒(IAV)M2的野生型(WT)信道以及在细胞培养中的病毒复制敏感探针。使用电生理学(EP)测量的M2 S31N通道的结合动力学非常依赖于在2和3中实现的金刚烷部分与芳基首基的第一环之间的长度以及在4和5中实现的金刚烷加合物的周长和长度。研究1 – 6表明,根据分子动力学(MD)模拟和分子力学泊松-玻尔兹曼表面积(MM / PBSA)计算,所有分子均在M2 S31N通道中结合,金刚烷基位于V27和G34之间,而芳基则突出于苯基(或6中的异恶唑)嵌入V27簇中的通道。在该向外结合构型中,金刚烷胺2中仅一种亚甲基的配体伸长或分别在4和5中使用金刚烷或金刚烷而不是金刚烷的配体伸长导致不完全进入并促进退出,与金刚烷胺衍生物1和2相比,消除了有效的嵌段。6。在活动的M2 S31N阻止器中1和6,苯基和异恶唑基头部基团实现更深的装订位置和高ķ上/低ķ关闭和高ķ上/高ķ离速率常数相比,不活动的2 - 5,其具有低得多的ķ上和更高ķ关。化合物1 - 5块由在从V27-H37的较长区域结合,在向内取向M2 WT信道,具有高ķ上和低ķ关闭速率常数。通过含有M2 WT或M2 S31N流感病毒的细胞培养物的感染是通过抑制1 - 5或1 - 4和6分别。而1和6块感染通过在S31N的变体M2块机构,2 - 4可通过溶酶体效应阻断在细胞培养物M2 S31N病毒复制,正如氯喹被认为能够抑制SARS-CoV的-2感染。



























 京公网安备 11010802027423号
京公网安备 11010802027423号