当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Serial Femtosecond Zero Dose Crystallography Captures a Water-Free Distal Heme Site in a Dye-Decolorising Peroxidase to Reveal a Catalytic Role for an Arginine in FeIV =O Formation.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-08-11 , DOI: 10.1002/anie.202008622 Marina Lučić 1 , Dimitri A Svistunenko 1 , Michael T Wilson 1 , Amanda K Chaplin 1 , Bradley Davy 2 , Ali Ebrahim 1, 2 , Danny Axford 2 , Takehiko Tosha 3 , Hiroshi Sugimoto 3 , Shigeki Owada 3, 4 , Florian S N Dworkowski 5 , Ivo Tews 6 , Robin L Owen 2 , Michael A Hough 1 , Jonathan A R Worrall 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-08-11 , DOI: 10.1002/anie.202008622 Marina Lučić 1 , Dimitri A Svistunenko 1 , Michael T Wilson 1 , Amanda K Chaplin 1 , Bradley Davy 2 , Ali Ebrahim 1, 2 , Danny Axford 2 , Takehiko Tosha 3 , Hiroshi Sugimoto 3 , Shigeki Owada 3, 4 , Florian S N Dworkowski 5 , Ivo Tews 6 , Robin L Owen 2 , Michael A Hough 1 , Jonathan A R Worrall 1
Affiliation
|
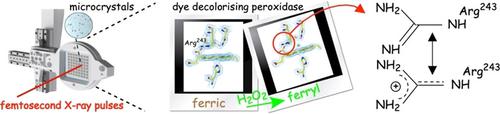
Obtaining structures of intact redox states of metal centers derived from zero dose X‐ray crystallography can advance our mechanistic understanding of metalloenzymes. In dye‐decolorising heme peroxidases (DyPs), controversy exists regarding the mechanistic role of the distal heme residues aspartate and arginine in the heterolysis of peroxide to form the catalytic intermediate compound I (FeIV=O and a porphyrin cation radical). Using serial femtosecond X‐ray crystallography (SFX), we have determined the pristine structures of the FeIII and FeIV=O redox states of a B‐type DyP. These structures reveal a water‐free distal heme site that, together with the presence of an asparagine, imply the use of the distal arginine as a catalytic base. A combination of mutagenesis and kinetic studies corroborate such a role. Our SFX approach thus provides unique insight into how the distal heme site of DyPs can be tuned to select aspartate or arginine for the rate enhancement of peroxide heterolysis.
中文翻译:

系列飞秒零剂量晶体学捕获染料脱色过氧化物酶中的无水远端血红素位点,揭示精氨酸在 FeIV =O 形成中的催化作用。
从零剂量 X 射线晶体学获得金属中心完整氧化还原态的结构可以促进我们对金属酶的机理理解。在染料脱色血红素过氧化物酶 (DyPs) 中,关于远端血红素残基天冬氨酸和精氨酸在过氧化物杂解形成催化中间体化合物 I(Fe IV =O 和卟啉阳离子自由基)中的机制作用存在争议。使用系列飞秒 X 射线晶体学 (SFX),我们确定了B 型 DyP 的Fe III和 Fe IV =O 氧化还原态的原始结构。这些结构揭示了一个无水的远端血红素位点,再加上天冬酰胺的存在,意味着使用远端精氨酸作为催化碱。诱变和动力学研究的结合证实了这种作用。因此,我们的 SFX 方法提供了关于如何调整 DyP 的远端血红素位点以选择天冬氨酸或精氨酸来增强过氧化物异解速率的独特见解。
更新日期:2020-08-11
中文翻译:

系列飞秒零剂量晶体学捕获染料脱色过氧化物酶中的无水远端血红素位点,揭示精氨酸在 FeIV =O 形成中的催化作用。
从零剂量 X 射线晶体学获得金属中心完整氧化还原态的结构可以促进我们对金属酶的机理理解。在染料脱色血红素过氧化物酶 (DyPs) 中,关于远端血红素残基天冬氨酸和精氨酸在过氧化物杂解形成催化中间体化合物 I(Fe IV =O 和卟啉阳离子自由基)中的机制作用存在争议。使用系列飞秒 X 射线晶体学 (SFX),我们确定了B 型 DyP 的Fe III和 Fe IV =O 氧化还原态的原始结构。这些结构揭示了一个无水的远端血红素位点,再加上天冬酰胺的存在,意味着使用远端精氨酸作为催化碱。诱变和动力学研究的结合证实了这种作用。因此,我们的 SFX 方法提供了关于如何调整 DyP 的远端血红素位点以选择天冬氨酸或精氨酸来增强过氧化物异解速率的独特见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号