当前位置:
X-MOL 学术
›
Enzyme Microb. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational design of new enzymes for hydrolysis and synthesis of third-generation cephalosporin antibiotics
Enzyme and Microbial Technology ( IF 3.4 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.enzmictec.2020.109649 Jing Xue 1 , Pengyu Wang 1 , Jianyong Kuang 1 , Yushan Zhu 2
Enzyme and Microbial Technology ( IF 3.4 ) Pub Date : 2020-10-01 , DOI: 10.1016/j.enzmictec.2020.109649 Jing Xue 1 , Pengyu Wang 1 , Jianyong Kuang 1 , Yushan Zhu 2
Affiliation
|
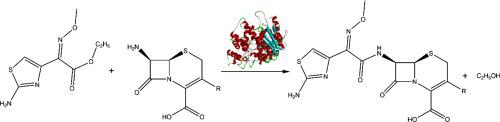
Engineering active sites in inert scaffolds to catalyze chemical transformations with unnatural substrates is still a great challenge for enzyme catalysis. In this research, a p-nitrobenzyl esterase from Bacillus subtilis was identified from the structural database, and a double mutant E115A/E188A was designed to afford catalytic activities toward the hydrolysis of ceftizoxime. A quadruple mutant E115A/E188A/L362S/I270A with enhanced catalytic efficiency was created to catalyze the condensation reaction of ethyl-2-methoxy-amino-2-(2-aminothiazole-4-yl) acetate with 7-amino-3-nor-cephalosporanic acid to produce ceftizoxime in a fully aqueous medium. The catalytic efficiencies of the computationally designed mutants E115A/E188A/L362S/I270A and E115A/Y118 K/E188 V/I270A/L362S can be taken as starting points to further improve their properties towards the practical application in designing more ecology-friendly production of third-generation cephalosporins.
中文翻译:

第三代头孢菌素类抗生素水解合成新酶的计算设计
在惰性支架中设计活性位点以催化非天然底物的化学转化仍然是酶催化的一大挑战。在这项研究中,从结构数据库中鉴定了来自枯草芽孢杆菌的对硝基苄酯酶,并设计了双突变体 E115A/E188A 以提供对头孢唑肟水解的催化活性。制备了具有增强催化效率的四重突变体 E115A/E188A/L362S/I270A 以催化 2-甲氧基-氨基-2-(2-氨基噻唑-4-基)乙酸乙酯与 7-氨基-3-nor 的缩合反应。 - 头孢烷酸在全水性介质中生产头孢唑肟。
更新日期:2020-10-01
中文翻译:

第三代头孢菌素类抗生素水解合成新酶的计算设计
在惰性支架中设计活性位点以催化非天然底物的化学转化仍然是酶催化的一大挑战。在这项研究中,从结构数据库中鉴定了来自枯草芽孢杆菌的对硝基苄酯酶,并设计了双突变体 E115A/E188A 以提供对头孢唑肟水解的催化活性。制备了具有增强催化效率的四重突变体 E115A/E188A/L362S/I270A 以催化 2-甲氧基-氨基-2-(2-氨基噻唑-4-基)乙酸乙酯与 7-氨基-3-nor 的缩合反应。 - 头孢烷酸在全水性介质中生产头孢唑肟。









































 京公网安备 11010802027423号
京公网安备 11010802027423号