Cell Reports Physical Science ( IF 7.9 ) Pub Date : 2020-08-12 , DOI: 10.1016/j.xcrp.2020.100145 Zheng Zhang , Liang Yu , Yunchuan Tu , Ruixue Chen , Lihui Wu , Junfa Zhu , Dehui Deng
|
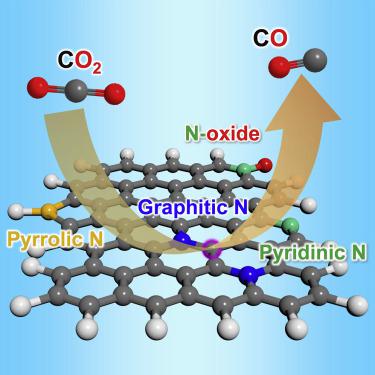
Nitrogen-doped (N-doped) carbon materials have been widely studied for electrocatalytic CO2R to CO. However, the active sites in N-doped carbon remain under debate owing to the complication in N types and the challenge in controllable synthesis. Here, via an innovative approach of template-assisted pyrolysis of phthalocyanine, we achieve a controlled preparation of N types in N-doped carbon foams. Electrochemical experiments show that the catalyst dominated by graphitic N rather than other N types drives highly selective CO2R to CO against the hydrogen evolution reaction, which achieves a CO Faradaic efficiency of 95% at −0.5 V versus RHE and runs stably for 80 h. Theoretical calculations indicate that carbon atoms next to graphitic N are triggered for the CO production, while carbon atoms next to pyridinic N promote the hydrogen evolution and pyrrolic N disfavors both reactions.
中文翻译:

公开用于电催化还原二氧化碳的无金属氮掺杂碳的活性位点
氮掺杂(N掺杂)碳材料已被广泛用于将CO 2 R转化为CO的电催化研究。然而,由于N型化合物的复杂性和可控合成的挑战,氮掺杂碳中的活性位仍然存在争议。在这里,通过模板辅助酞菁热解的创新方法,我们实现了N掺杂碳泡沫中N型的可控制备。电化学实验表明,以石墨氮而不是其他氮为主的催化剂可驱动高选择性的CO 2R转化为CO对抗氢逸出反应,相对于RHE,在-0.5 V时可实现95%的CO法拉第效率,并稳定运行80 h。理论计算表明,石墨N旁边的碳原子被触发生成CO,而吡啶N旁边的碳原子则促进氢的释放,而吡咯N则不利于这两个反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号