当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct Measurement of Single-Molecule Ligand-Receptor Interactions.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-08-11 , DOI: 10.1021/acs.jpcb.0c05474 K-T Lam 1 , E L Taylor 1 , A J Thompson 2 , M-D Ruepp 3, 4 , M Lochner 4 , Michael J Martinez 1 , J A Brozik 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2020-08-11 , DOI: 10.1021/acs.jpcb.0c05474 K-T Lam 1 , E L Taylor 1 , A J Thompson 2 , M-D Ruepp 3, 4 , M Lochner 4 , Michael J Martinez 1 , J A Brozik 1
Affiliation
|
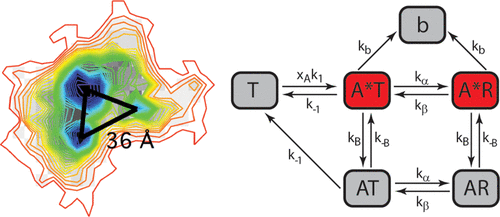
Measuring the kinetics that govern ligand–receptor interactions is fundamental to our understanding of pharmacology. For ligand-gated ion channels, binding of an agonist triggers allosteric motions that open an integral ion-permeable pore. By mathematically modeling stochastic electrophysiological responses with high temporal resolution (ms), previous single channel studies have been able to infer the rate constants of ligands binding to these receptors. However, there are no reports of the direct measurement of the single-molecule binding events that are vital to how agonists exert their functional effects. For the first time, we report these direct measurements, the rate constants, and corresponding free energy changes, which describe the transitions between the different binding states. To achieve this, we use the super resolution technique: points accumulation for imaging in nanoscale topography (PAINT) to observe binding of ATP to orthosteric binding sites on the P2X1 receptor. Furthermore, an analysis of time-resolved single-molecule interactions is used to measure elementary rate constants and thermodynamic forces that drive the allosteric motions. These single-molecule measurements unequivocally establish the location of each binding states of the P2X1 receptor and the stochastic nature of the interaction with its native ligand. The analysis leads to the measurement of the forward and reverse rates from a weak ligand-binding state to a strong ligand binding state that is linked to allosteric motion and ion pore formation. These rates (kα = 1.41 sec–1 and kβ = 0.32 sec–1) were then used to determine the free energy associated with this critical mechanistic step (3.7 kJ/mol). Importantly, the described methods can be readily applied to all ligand-gated ion channels, and more broadly to the molecular interactions of other classes of membrane proteins.
中文翻译:

直接测量单分子配体-受体相互作用。
测量控制配体-受体相互作用的动力学是理解药理学的基础。对于配体门控离子通道,激动剂的结合会触发变构运动,从而打开一个完整的离子可渗透孔。通过数学建模具有高时间分辨率(ms)的随机电生理响应,以前的单通道研究已经能够推断出与这些受体结合的配体的速率常数。但是,尚无直接测量单分子结合事件的报道,这对于激动剂如何发挥其功能作用至关重要。我们首次报告了这些直接测量值,速率常数和相应的自由能变化,这些变化描述了不同结合状态之间的过渡。为此,我们使用了超分辨率技术:在纳米尺度地形学(PAINT)中成像的积分点,以观察ATP与P2X1受体的正构结合位点的结合。此外,对时间分辨的单分子相互作用的分析用于测量基本速率常数和驱动变构运动的热力学力。这些单分子测量明确地确定了P2X1受体每个结合状态的位置以及与它的天然配体相互作用的随机性。该分析导致对从变构运动和离子孔形成相关的从弱配体结合状态到强配体结合状态的正向和反向速率的测量。这些费率(此外,对时间分辨的单分子相互作用的分析用于测量基本速率常数和驱动变构运动的热力学力。这些单分子测量明确地确定了P2X1受体每个结合状态的位置以及与它的天然配体相互作用的随机性质。该分析导致对从变构运动和离子孔形成相关的从弱配体结合状态到强配体结合状态的正向和反向速率的测量。这些费率(此外,对时间分辨的单分子相互作用的分析用于测量基本速率常数和驱动变构运动的热力学力。这些单分子测量明确地确定了P2X1受体每个结合状态的位置以及与它的天然配体相互作用的随机性质。该分析导致对从变构运动和离子孔形成相关的从弱配体结合状态到强配体结合状态的正向和反向速率的测量。这些费率(这些单分子测量明确地确定了P2X1受体每个结合状态的位置以及与它的天然配体相互作用的随机性质。该分析导致对从变构运动和离子孔形成相关的从弱配体结合状态到强配体结合状态的正向和反向速率的测量。这些费率(这些单分子测量明确地确定了P2X1受体每个结合状态的位置以及与它的天然配体相互作用的随机性质。该分析导致对从变构运动和离子孔形成相关的从弱配体结合状态到强配体结合状态的正向和反向速率的测量。这些费率(ķ α = 1.41秒-1和ķ β = 0.32秒-1)然后用来确定与这一关键机制步骤(3.7千焦/摩尔)相关联的自由能。重要的是,所描述的方法可以容易地应用于所有配体门控的离子通道,并且更广泛地应用于其他类型的膜蛋白的分子相互作用。
更新日期:2020-09-10
中文翻译:

直接测量单分子配体-受体相互作用。
测量控制配体-受体相互作用的动力学是理解药理学的基础。对于配体门控离子通道,激动剂的结合会触发变构运动,从而打开一个完整的离子可渗透孔。通过数学建模具有高时间分辨率(ms)的随机电生理响应,以前的单通道研究已经能够推断出与这些受体结合的配体的速率常数。但是,尚无直接测量单分子结合事件的报道,这对于激动剂如何发挥其功能作用至关重要。我们首次报告了这些直接测量值,速率常数和相应的自由能变化,这些变化描述了不同结合状态之间的过渡。为此,我们使用了超分辨率技术:在纳米尺度地形学(PAINT)中成像的积分点,以观察ATP与P2X1受体的正构结合位点的结合。此外,对时间分辨的单分子相互作用的分析用于测量基本速率常数和驱动变构运动的热力学力。这些单分子测量明确地确定了P2X1受体每个结合状态的位置以及与它的天然配体相互作用的随机性。该分析导致对从变构运动和离子孔形成相关的从弱配体结合状态到强配体结合状态的正向和反向速率的测量。这些费率(此外,对时间分辨的单分子相互作用的分析用于测量基本速率常数和驱动变构运动的热力学力。这些单分子测量明确地确定了P2X1受体每个结合状态的位置以及与它的天然配体相互作用的随机性质。该分析导致对从变构运动和离子孔形成相关的从弱配体结合状态到强配体结合状态的正向和反向速率的测量。这些费率(此外,对时间分辨的单分子相互作用的分析用于测量基本速率常数和驱动变构运动的热力学力。这些单分子测量明确地确定了P2X1受体每个结合状态的位置以及与它的天然配体相互作用的随机性质。该分析导致对从变构运动和离子孔形成相关的从弱配体结合状态到强配体结合状态的正向和反向速率的测量。这些费率(这些单分子测量明确地确定了P2X1受体每个结合状态的位置以及与它的天然配体相互作用的随机性质。该分析导致对从变构运动和离子孔形成相关的从弱配体结合状态到强配体结合状态的正向和反向速率的测量。这些费率(这些单分子测量明确地确定了P2X1受体每个结合状态的位置以及与它的天然配体相互作用的随机性质。该分析导致对从变构运动和离子孔形成相关的从弱配体结合状态到强配体结合状态的正向和反向速率的测量。这些费率(ķ α = 1.41秒-1和ķ β = 0.32秒-1)然后用来确定与这一关键机制步骤(3.7千焦/摩尔)相关联的自由能。重要的是,所描述的方法可以容易地应用于所有配体门控的离子通道,并且更广泛地应用于其他类型的膜蛋白的分子相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号