当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and Validation of Liposomal ApoE2 Gene Delivery System to Evade Blood–Brain Barrier for Effective Treatment of Alzheimer’s Disease
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-08-11 , DOI: 10.1021/acs.molpharmaceut.0c00461 Sanjay Arora 1 , Buddhadev Layek 1 , Jagdish Singh 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2020-08-11 , DOI: 10.1021/acs.molpharmaceut.0c00461 Sanjay Arora 1 , Buddhadev Layek 1 , Jagdish Singh 1
Affiliation
|
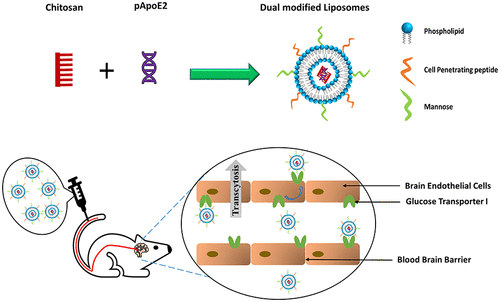
Targeting gene-based therapeutics to the brain is a strategy actively sought to treat Alzheimer’s disease (AD). Recent findings discovered the role of apolipoprotein E (ApoE) isoforms in the clearance of toxic amyloid beta proteins from the brain. ApoE2 isoform is beneficial for preventing AD development, whereas ApoE4 is a major contributing factor to the disease. In this paper, we demonstrated efficient brain-targeted delivery of ApoE2 encoding plasmid DNA (pApoE2) using glucose transporter-1 (glut-1) targeted liposomes. Liposomes were surface-functionalized with a glut-1 targeting ligand mannose (MAN) and a cell-penetrating peptide (CPP) to enhance brain-targeting and cellular internalization, respectively. Among various CPPs, rabies virus glycoprotein peptide (RVG) or penetratin (Pen) was selected as a cell-penetration enhancer. Dual (RVGMAN and PenMAN)-functionalized liposomes were cytocompatible at 100 nM phospholipid concentration and demonstrated significantly higher expression of ApoE2 in bEnd.3 cells, primary neurons, and astrocytes compared to monofunctionalized and unmodified (plain) liposomes. Dual-modified liposomes also showed ∼2 times higher protein expression than other formulation controls in neurons cultured below the in vitro BBB model. These results translated well to in vivo efficacy study with significantly higher transfection of pApoE2 in the C57BL/6 mice brain following single tail vein administration of RVGMAN and PenMAN functionalized liposomes without any noticeable signs of toxicity. These results illustrate the potential of surface-modified liposomes for safe and brain-targeted delivery of the pApoE2 gene for effective AD therapy.
中文翻译:

绕过血脑屏障有效治疗阿尔茨海默病的脂质体 ApoE2 基因传递系统的设计和验证
针对大脑进行基于基因的治疗是一种积极寻求治疗阿尔茨海默病 (AD) 的策略。最近的研究结果发现了载脂蛋白 E (ApoE) 同工型在清除大脑中有毒的淀粉样β蛋白中的作用。 ApoE2 同工型有利于预防 AD 的发展,而 ApoE4 是导致该疾病的主要因素。在本文中,我们证明了使用葡萄糖转运蛋白 1 (glut-1) 靶向脂质体有效地脑靶向递送 ApoE2 编码质粒 DNA (pApoE2)。脂质体用 glut-1 靶向配体甘露糖 (MAN) 和细胞穿透肽 (CPP) 进行表面功能化,以分别增强脑靶向和细胞内化。在各种CPP中,选择狂犬病病毒糖蛋白肽(RVG)或渗透素(Pen)作为细胞渗透增强剂。双功能化脂质体(RVGMAN 和 PenMAN)在 100 nM 磷脂浓度下具有细胞相容性,并且与单功能化和未修饰(普通)脂质体相比,bEnd.3 细胞、原代神经元和星形胶质细胞中 ApoE2 的表达显着更高。在体外BBB 模型下培养的神经元中,双修饰脂质体的蛋白质表达量也比其他制剂对照高约 2 倍。这些结果很好地转化为体内功效研究,单尾静脉施用 RVGMAN 和 PenMAN 功能化脂质体后,C57BL/6 小鼠大脑中 pApoE2 的转染率显着升高,且没有任何明显的毒性迹象。这些结果说明了表面修饰脂质体在安全和脑靶向递送 pApoE2 基因以有效治疗 AD 方面的潜力。
更新日期:2020-08-11
中文翻译:

绕过血脑屏障有效治疗阿尔茨海默病的脂质体 ApoE2 基因传递系统的设计和验证
针对大脑进行基于基因的治疗是一种积极寻求治疗阿尔茨海默病 (AD) 的策略。最近的研究结果发现了载脂蛋白 E (ApoE) 同工型在清除大脑中有毒的淀粉样β蛋白中的作用。 ApoE2 同工型有利于预防 AD 的发展,而 ApoE4 是导致该疾病的主要因素。在本文中,我们证明了使用葡萄糖转运蛋白 1 (glut-1) 靶向脂质体有效地脑靶向递送 ApoE2 编码质粒 DNA (pApoE2)。脂质体用 glut-1 靶向配体甘露糖 (MAN) 和细胞穿透肽 (CPP) 进行表面功能化,以分别增强脑靶向和细胞内化。在各种CPP中,选择狂犬病病毒糖蛋白肽(RVG)或渗透素(Pen)作为细胞渗透增强剂。双功能化脂质体(RVGMAN 和 PenMAN)在 100 nM 磷脂浓度下具有细胞相容性,并且与单功能化和未修饰(普通)脂质体相比,bEnd.3 细胞、原代神经元和星形胶质细胞中 ApoE2 的表达显着更高。在体外BBB 模型下培养的神经元中,双修饰脂质体的蛋白质表达量也比其他制剂对照高约 2 倍。这些结果很好地转化为体内功效研究,单尾静脉施用 RVGMAN 和 PenMAN 功能化脂质体后,C57BL/6 小鼠大脑中 pApoE2 的转染率显着升高,且没有任何明显的毒性迹象。这些结果说明了表面修饰脂质体在安全和脑靶向递送 pApoE2 基因以有效治疗 AD 方面的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号