当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Perfusion Cultivation of Artificial Liver Extracellular Matrix in Fibrous Polymer Sponges Biomimicking Scaffolds for Tissue Engineering.
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-08-11 , DOI: 10.1021/acs.biomac.0c00900 Michael Mader 1 , Moritz Helm 2 , Mingxia Lu 3 , Martina H Stenzel 3 , Valérie Jérôme 2 , Ruth Freitag 2 , Seema Agarwal 1 , Andreas Greiner 1
Biomacromolecules ( IF 5.5 ) Pub Date : 2020-08-11 , DOI: 10.1021/acs.biomac.0c00900 Michael Mader 1 , Moritz Helm 2 , Mingxia Lu 3 , Martina H Stenzel 3 , Valérie Jérôme 2 , Ruth Freitag 2 , Seema Agarwal 1 , Andreas Greiner 1
Affiliation
|
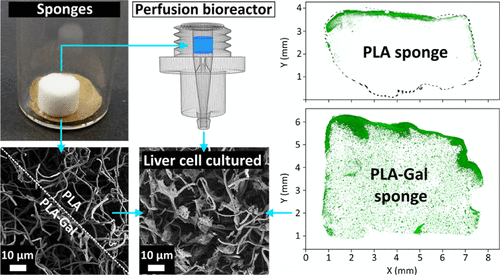
A major challenge in tissue engineering and artificial scaffolding is to combine easily tunable scaffolds biomimicking the extracellular matrix of native organs with delivery-controlled cell culturing to create fully cellularized, large artificial 3D scaffolds. Aiming at bioartificial liver construction, we present our research using galactose-functionalized, ultraporous polylactide 3D nanofiber sponges fabricated out of electrospun fibers. Sponge biomodification by blend galactosylation and in-solution coating is performed, respectively, using a polylactide-galactose carrier-copolymer that promotes cell delivery and features a pronounced autofluorescence. It allows us to verify the galactosylation success, evaluate its quality, and record dye-free, high-resolution images of the sponge network using confocal laser scanning microscopy. The galactose carrier and its impact on scaffold cellularization is validated in benchmark to several reference systems. Verification of the human hepatic cell asialoglycoprotein receptor presence and galactose interaction in culture is performed by Cu2+ receptor-blocking experiments. The culture results are extensively investigated in and ex situ to trace and quantify the cell culture progress, cell activity, and viability at different culture stages. Bioreactor cultivation of sponges reveals that the galactose carrier does not only facilitate cell adhesion but also enhances cellular distribution throughout the scaffold. The promising 3D culture results allow us to move forward to create mature in vitro liver model research systems. The elaboration into ex vivo testing platforms could help judging native cell material interactions with drugs or therapeutics, without the need of direct human or animal testing.
中文翻译:

在组织工程用纤维聚合物海绵仿生支架中灌注培养人工肝细胞外基质。
组织工程和人工支架的主要挑战是将模仿自然器官的细胞外基质的易于调节的支架与递送控制的细胞培养相结合,以创建完全细胞化的大型人工3D支架。针对生物人工肝的构造,我们介绍了使用半乳糖功能化,由电纺纤维制成的超多孔聚丙交酯3D纳米纤维海绵进行的研究。使用能促进细胞递送并具有明显自发荧光功能的聚丙交酯-半乳糖载体-共聚物分别进行混合半乳糖基化和溶液内包被的海绵生物改性。它使我们能够验证半乳糖基化成功,评估其质量,并使用共聚焦激光扫描显微镜记录海绵网络的无染料高分辨率图像。半乳糖载体及其对支架细胞化的影响已在一些参考系统的基准中得到验证。Cu对人肝细胞脱唾液酸糖蛋白受体的存在和半乳糖相互作用的验证是通过Cu进行的2+受体阻断实验。对培养结果进行了广泛的内部和异地研究,以追踪和量化不同培养阶段的细胞培养进度,细胞活性和活力。海绵的生物反应器培养表明,半乳糖载体不仅促进细胞粘附,而且还增强了整个支架中的细胞分布。令人鼓舞的3D培养结果使我们能够前进,以创建成熟的体外肝模型研究系统。精心设计成离体测试平台可以帮助判断天然细胞材料与药物或治疗剂的相互作用,而无需直接进行人或动物测试。
更新日期:2020-10-12
中文翻译:

在组织工程用纤维聚合物海绵仿生支架中灌注培养人工肝细胞外基质。
组织工程和人工支架的主要挑战是将模仿自然器官的细胞外基质的易于调节的支架与递送控制的细胞培养相结合,以创建完全细胞化的大型人工3D支架。针对生物人工肝的构造,我们介绍了使用半乳糖功能化,由电纺纤维制成的超多孔聚丙交酯3D纳米纤维海绵进行的研究。使用能促进细胞递送并具有明显自发荧光功能的聚丙交酯-半乳糖载体-共聚物分别进行混合半乳糖基化和溶液内包被的海绵生物改性。它使我们能够验证半乳糖基化成功,评估其质量,并使用共聚焦激光扫描显微镜记录海绵网络的无染料高分辨率图像。半乳糖载体及其对支架细胞化的影响已在一些参考系统的基准中得到验证。Cu对人肝细胞脱唾液酸糖蛋白受体的存在和半乳糖相互作用的验证是通过Cu进行的2+受体阻断实验。对培养结果进行了广泛的内部和异地研究,以追踪和量化不同培养阶段的细胞培养进度,细胞活性和活力。海绵的生物反应器培养表明,半乳糖载体不仅促进细胞粘附,而且还增强了整个支架中的细胞分布。令人鼓舞的3D培养结果使我们能够前进,以创建成熟的体外肝模型研究系统。精心设计成离体测试平台可以帮助判断天然细胞材料与药物或治疗剂的相互作用,而无需直接进行人或动物测试。











































 京公网安备 11010802027423号
京公网安备 11010802027423号