当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformational Dynamics of the Periplasmic Chaperone SurA.
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-11 , DOI: 10.1021/acs.biochem.0c00507 Moye Jia 1 , Bo Wu 2 , Ziyu Yang 3, 4 , Chunlai Chen 2 , Meiping Zhao 3, 4 , Xianhui Hou 1 , Xiaogang Niu 1 , Changwen Jin 1, 3 , Yunfei Hu 5, 6
Biochemistry ( IF 2.9 ) Pub Date : 2020-08-11 , DOI: 10.1021/acs.biochem.0c00507 Moye Jia 1 , Bo Wu 2 , Ziyu Yang 3, 4 , Chunlai Chen 2 , Meiping Zhao 3, 4 , Xianhui Hou 1 , Xiaogang Niu 1 , Changwen Jin 1, 3 , Yunfei Hu 5, 6
Affiliation
|
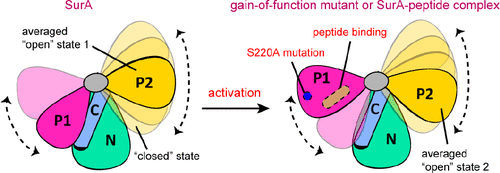
The periplasmic protein SurA is the primary chaperone involved in the biogenesis of bacterial outer membrane proteins and is a potential antibacterial drug target. The three-dimensional structure of SurA can be divided into three parts, a core module formed by the N- and C-terminal regions and two peptidyl-prolyl isomerase (PPIase) domains, P1 and P2. Despite the determination of the structures of several SurA–peptide complexes, the functional mechanism of this chaperone remains elusive and the roles of the two PPIase domains are yet unclear. Herein, we characterize the conformational dynamics of SurA by using solution nuclear magnetic resonance and single-molecule fluorescence resonance energy transfer methods. We demonstrate a “closed-to-open” structural transition of the P1 domain that is correlated with both chaperone activity and peptide binding and show that the flexible P2 domain can also occupy conformations that closely contact the NC core module. Our results offer a structural basis for the counteracting roles of the two PPIase domains in regulating the SurA chaperone activity.
中文翻译:

周质伴侣SurA的构象动力学。
周质蛋白SurA是参与细菌外膜蛋白生物发生的主要伴侣蛋白,并且是潜在的抗菌药物靶标。SurA的三维结构可分为三个部分,一个是由N和C端区域形成的核心模块,另一个是两个肽基脯氨酰异构酶(PPIase)结构域P1和P2。尽管已经确定了几种SurA-肽复合物的结构,但该分子伴侣的功能机制仍然不清楚,并且两个PPIase结构域的作用尚不清楚。在这里,我们通过使用溶液核磁共振和单分子荧光共振能量转移方法表征SurA的构象动力学。我们证明了与伴侣活性和肽结合相关的P1结构域的“封闭-开放”结构转变,并表明柔性P2结构域也可以占据紧密接触NC核心模块的构象。我们的结果为两个PPIase结构域在调节SurA伴侣活性中的抵消作用提供了结构基础。
更新日期:2020-09-08
中文翻译:

周质伴侣SurA的构象动力学。
周质蛋白SurA是参与细菌外膜蛋白生物发生的主要伴侣蛋白,并且是潜在的抗菌药物靶标。SurA的三维结构可分为三个部分,一个是由N和C端区域形成的核心模块,另一个是两个肽基脯氨酰异构酶(PPIase)结构域P1和P2。尽管已经确定了几种SurA-肽复合物的结构,但该分子伴侣的功能机制仍然不清楚,并且两个PPIase结构域的作用尚不清楚。在这里,我们通过使用溶液核磁共振和单分子荧光共振能量转移方法表征SurA的构象动力学。我们证明了与伴侣活性和肽结合相关的P1结构域的“封闭-开放”结构转变,并表明柔性P2结构域也可以占据紧密接触NC核心模块的构象。我们的结果为两个PPIase结构域在调节SurA伴侣活性中的抵消作用提供了结构基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号