当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Specific Key-Point Mutations along the Helical Conformation of Huntingtin-Exon 1 Protein Might Have an Antagonistic Effect on the Toxic Helical Content's Formation.
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-08-10 , DOI: 10.1021/acschemneuro.0c00493 Sanda Nastasia Moldovean 1 , Vasile Chiş 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2020-08-10 , DOI: 10.1021/acschemneuro.0c00493 Sanda Nastasia Moldovean 1 , Vasile Chiş 1
Affiliation
|
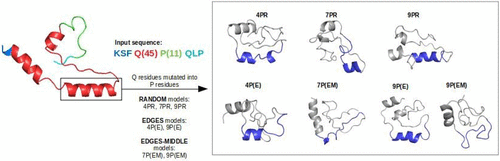
The polyglutamine tract length represents a key regulator for the Huntington’s disease toxicity level and its aggregation rates, often being related to helical structural conformations. In this study, we performed all-atom MD simulations on mutant Huntingtin-Exon1 protein with additional mutation spots, aiming to observe the corresponding structural and dynamical changes at the level of the helix. The simulated structures consist of three sets of Q residue mutations into P residues (4P, 7P, and 9P), with each set including different spots of mutations: random along the mutant sequence (R models), at the edges of the helix (E models), as well as at the edges and in the middle of the helix (EM models). At the helical level, our results predict less compactness profiles for a higher number of P mutations (7P and 9P models) with particular mutation spots at the edges and at the edges-middle of the helix. Moreover, the C-alpha atom distances decreased for 7P and 9P models in comparison to 4P models, and the RMSF values show the highest fluctuation rates for 9P models with point mutations at the edges and in the middle of the helix. The secondary structure analysis suggests greater structural transitions from α-helices to bends, turns, and random coils for 7P and 9P models, particularly for point mutations considered at the edges and in the middle of the helical content. The obtained results support our hypothesis that specific key-point mutations along the helical conformation might have an antagonistic effect on the toxic helical content’s formation.
中文翻译:

沿着Huntingtin-Exon 1蛋白的螺旋构象的特定关键点突变可能对有毒螺旋含量的形成具有拮抗作用。
聚谷氨酰胺束长度是亨廷顿氏病毒性水平及其聚集率的关键调节因子,通常与螺旋结构构象有关。在这项研究中,我们对带有额外突变点的突变Huntingtin-Exon1蛋白进行了全原子MD模拟,旨在观察螺旋水平上相应的结构和动力学变化。模拟的结构由三组Q残基突变为P残基(4P,7P和9P)组成,每组包括不同的突变点:沿突变序列随机(R模型),在螺旋的边缘(E型号),以及螺旋的边缘和中间(EM型号)。在螺旋层次上 我们的结果预测,对于较高数量的P突变(7P和9P模型),在螺旋的边缘和边缘的中间以及中间的特定突变点处,紧密度分布会较小。此外,与4P模型相比,7P和9P模型的C-α原子距离减小,并且RMSF值显示了在螺旋边缘和中间具有点突变的9P模型的最高波动率。二级结构分析表明,对于7P和9P模型,尤其是考虑到在螺旋内容的边缘和中间处的点突变,从α螺旋到弯曲,转弯和随机线圈的结构过渡更大。获得的结果支持我们的假设,即沿螺旋构象的特定关键点突变可能对有毒螺旋成分的形成具有拮抗作用。
更新日期:2020-09-16
中文翻译:

沿着Huntingtin-Exon 1蛋白的螺旋构象的特定关键点突变可能对有毒螺旋含量的形成具有拮抗作用。
聚谷氨酰胺束长度是亨廷顿氏病毒性水平及其聚集率的关键调节因子,通常与螺旋结构构象有关。在这项研究中,我们对带有额外突变点的突变Huntingtin-Exon1蛋白进行了全原子MD模拟,旨在观察螺旋水平上相应的结构和动力学变化。模拟的结构由三组Q残基突变为P残基(4P,7P和9P)组成,每组包括不同的突变点:沿突变序列随机(R模型),在螺旋的边缘(E型号),以及螺旋的边缘和中间(EM型号)。在螺旋层次上 我们的结果预测,对于较高数量的P突变(7P和9P模型),在螺旋的边缘和边缘的中间以及中间的特定突变点处,紧密度分布会较小。此外,与4P模型相比,7P和9P模型的C-α原子距离减小,并且RMSF值显示了在螺旋边缘和中间具有点突变的9P模型的最高波动率。二级结构分析表明,对于7P和9P模型,尤其是考虑到在螺旋内容的边缘和中间处的点突变,从α螺旋到弯曲,转弯和随机线圈的结构过渡更大。获得的结果支持我们的假设,即沿螺旋构象的特定关键点突变可能对有毒螺旋成分的形成具有拮抗作用。







































 京公网安备 11010802027423号
京公网安备 11010802027423号