当前位置:
X-MOL 学术
›
ChemPlusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Constitutionally Isomeric Aromatic Tripeptides: Self-Assembly and Metal-Ion-Modulated Transformations.
ChemPlusChem ( IF 3.0 ) Pub Date : 2020-08-11 , DOI: 10.1002/cplu.202000464 Narendra Singh 1 , Ramesh Singh 2 , Khashti Ballabh Joshi 2 , Sandeep Verma 1
ChemPlusChem ( IF 3.0 ) Pub Date : 2020-08-11 , DOI: 10.1002/cplu.202000464 Narendra Singh 1 , Ramesh Singh 2 , Khashti Ballabh Joshi 2 , Sandeep Verma 1
Affiliation
|
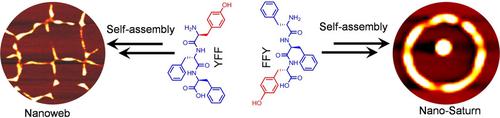
Self‐assembling peptides based on aromatic amino acids can adopt diverse nanostructures which primarily depend on their molecular structures. Therefore, to understand the nature of self‐assembly on the molecular level we rationally designed two constitutional isomers of short aromatic peptides. The first isomer consists of a tyrosine moiety at the N‐terminus and the second isomer consists of a tyrosine moiety at the C‐terminus of the FF peptide, a core recognition motif of Amyloid β peptides. Therefore, it can be considered that both the designed tripeptides are the analogues of the FFF peptide with only atomic(−H) level replacement by −OH functional group on the first and last phenyl ring, respectively. The first isomer self‐assembled into 2D porous nanosheets (“Nanowebs”), however the second isomers produced toroidal shapes with central spheres (“Nano‐Saturn” like assemblies). Interestingly, the presence of the transition‐metal ions (copper, zinc and iron) triggered the self‐assembly of both the peptides into fibrous circular discs, nanomats and nanoplates like assembly.
中文翻译:

体相异构的芳香三肽:自组装和金属离子调节的转变。
基于芳香族氨基酸的自组装肽可以采用多种纳米结构,这主要取决于其分子结构。因此,为了了解分子水平上自组装的本质,我们合理地设计了短芳香肽的两个结构异构体。第一个异构体由N末端的酪氨酸部分组成,第二个异构体由FF肽(淀粉样β肽的核心识别基序)的C末端的酪氨酸部分组成。因此,可以认为这两个设计的三肽都是FFF肽的类似物,仅第一个和最后一个苯环上的-OH官能团分别被原子(-H)级取代。第一个异构体自组装成二维多孔纳米片(“纳米网”),然而,第二种异构体产生了带有中心球体的环形形状(像“纳米土星”那样的组件)。有趣的是,过渡金属离子(铜,锌和铁)的存在触发了这两种肽的自组装,成为纤维圆盘,纳米垫和纳米板之类的组装。
更新日期:2020-09-02
中文翻译:

体相异构的芳香三肽:自组装和金属离子调节的转变。
基于芳香族氨基酸的自组装肽可以采用多种纳米结构,这主要取决于其分子结构。因此,为了了解分子水平上自组装的本质,我们合理地设计了短芳香肽的两个结构异构体。第一个异构体由N末端的酪氨酸部分组成,第二个异构体由FF肽(淀粉样β肽的核心识别基序)的C末端的酪氨酸部分组成。因此,可以认为这两个设计的三肽都是FFF肽的类似物,仅第一个和最后一个苯环上的-OH官能团分别被原子(-H)级取代。第一个异构体自组装成二维多孔纳米片(“纳米网”),然而,第二种异构体产生了带有中心球体的环形形状(像“纳米土星”那样的组件)。有趣的是,过渡金属离子(铜,锌和铁)的存在触发了这两种肽的自组装,成为纤维圆盘,纳米垫和纳米板之类的组装。











































 京公网安备 11010802027423号
京公网安备 11010802027423号