当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Posttranslationally Acting Arginases Provide a Ribosomal Route to Non-proteinogenic Ornithine Residues in Diverse Peptide Sequences.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-08-11 , DOI: 10.1002/anie.202008990 Silja Mordhorst 1 , Brandon I Morinaka 2 , Anna L Vagstad 1 , Jörn Piel 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-08-11 , DOI: 10.1002/anie.202008990 Silja Mordhorst 1 , Brandon I Morinaka 2 , Anna L Vagstad 1 , Jörn Piel 1
Affiliation
|
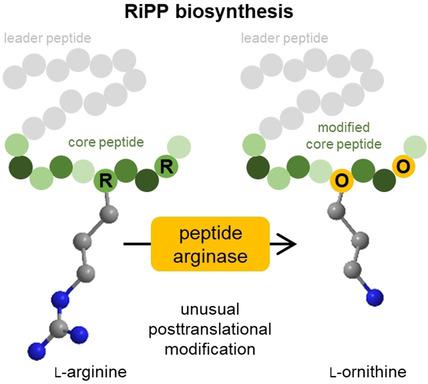
Ornithine is a component of many bioactive nonribosomal peptides but is challenging to incorporate into ribosomal products. We recently identified OspR, a cyanobacterial arginase‐like enzyme that installs ornithines in the antiviral ribosomally synthesised and posttranslationally modified peptide (RiPP) landornamide A. Here we report that OspR belongs to a larger family of peptide arginases from diverse organisms and RiPP types. In E. coli, seven selected enzymes converted arginine into ornithine with little preference for the leader type. A broad range of peptide sequences was modified, including polyarginine repeats. We also generated analogues of ornithine‐containing nonribosomal peptides using RiPP technology. Five pseudo‐nonribosomal products with ornithines at the correct positions were obtained, including a brevicidine analogue containing ornithine and a d‐amino acid installed by the peptide epimerase OspD. These results suggest new opportunities for peptide bioengineering.
中文翻译:

翻译后作用的精氨酸酶提供了一条核糖体途径,以多种肽序列的非蛋白鸟氨酸残基。
鸟氨酸是许多具有生物活性的非核糖体肽的组成部分,但要整合到核糖体产品中却具有挑战性。我们最近发现了OspR,一种蓝藻精氨酸酶样酶,可在抗病毒核糖体合成和翻译后修饰的肽(RiPP)兰德酰胺A中安装鸟苷。在大肠杆菌中,有7种选定的酶将精氨酸转化为鸟氨酸,而对前导类型则没有什么偏好。修饰了广泛的肽序列,包括聚精氨酸重复序列。我们还使用RiPP技术生成了含鸟氨酸的非核糖体肽的类似物。得到5个伪非核糖体产品在正确的位置鸟氨酸,包括含brevicidine类似物鸟氨酸和d由肽差向异构酶OSPD安装α-氨基酸。这些结果表明肽生物工程的新机会。
更新日期:2020-08-11
中文翻译:

翻译后作用的精氨酸酶提供了一条核糖体途径,以多种肽序列的非蛋白鸟氨酸残基。
鸟氨酸是许多具有生物活性的非核糖体肽的组成部分,但要整合到核糖体产品中却具有挑战性。我们最近发现了OspR,一种蓝藻精氨酸酶样酶,可在抗病毒核糖体合成和翻译后修饰的肽(RiPP)兰德酰胺A中安装鸟苷。在大肠杆菌中,有7种选定的酶将精氨酸转化为鸟氨酸,而对前导类型则没有什么偏好。修饰了广泛的肽序列,包括聚精氨酸重复序列。我们还使用RiPP技术生成了含鸟氨酸的非核糖体肽的类似物。得到5个伪非核糖体产品在正确的位置鸟氨酸,包括含brevicidine类似物鸟氨酸和d由肽差向异构酶OSPD安装α-氨基酸。这些结果表明肽生物工程的新机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号