Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantitative Delineation of the Low Energy Decomposition Pathway for Lithium Peroxide in Lithium–Oxygen Battery
Advanced Science ( IF 14.3 ) Pub Date : 2020-08-11 , DOI: 10.1002/advs.202001660 Arghya Dutta 1 , Kimihiko Ito 1 , Akihiro Nomura 1, 2 , Yoshimi Kubo 1, 2
Advanced Science ( IF 14.3 ) Pub Date : 2020-08-11 , DOI: 10.1002/advs.202001660 Arghya Dutta 1 , Kimihiko Ito 1 , Akihiro Nomura 1, 2 , Yoshimi Kubo 1, 2
Affiliation
|
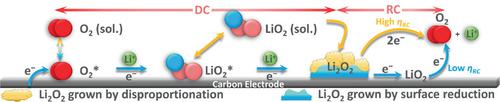
Identification of a low‐potential decomposition pathway for lithium peroxide (Li2O2) in nonaqueous lithium–oxygen (Li–O2) battery is urgently needed to ameliorate its poor energy efficiency. In this study, experimental data and theoretical calculations demonstrate that the recharge overpotential (ηRC) of Li–O2 battery is fundamentally dependent on the Li2O2 crystallization pathway which is intrinsically related to the microscopic structural properties of the growing crystals during discharge. The Li2O2 grown by concurrent surface reduction and chemical disproportionation seems to form two discrete phases that have been deconvoluted and the amount of Li2O2 deposited by these two routes is quantitatively estimated. Systematic analyses have demonstrated that, regardless of the bulk morphology, solution‐grown Li2O2 shows higher ηRC (>1 V) which can be attributed to higher structural order in the crystal compared to the surface‐grown Li2O2. Presumably due to a cohesive interaction between the electrode surface and growing crystals, the surface‐grown Li2O2 seems to possess microscopic structural disorder that facilitates a delithiation induced partial solution‐phase oxidation at lower ηRC (<0.5 V). This difference in ηRC for differently grown Li2O2 provides crucial insights into necessary control over Li2O2 crystallization pathways to improve the energy efficiency of a Li–O2 battery.
中文翻译:

锂氧电池中过氧化锂低能分解途径的定量描述
迫切需要确定非水锂氧(Li-O 2 )电池中过氧化锂(Li 2 O 2 )的低电势分解途径,以改善其较差的能源效率。在这项研究中,实验数据和理论计算表明,Li-O 2电池的再充电过电势( η RC )从根本上取决于Li 2 O 2 结晶途径,而Li 2 O 2结晶途径与放电过程中生长晶体的微观结构特性有着内在的联系。 。通过同时进行表面还原和化学歧化生长的Li 2 O 2似乎形成了两个已解卷积的离散相,并且定量估计了通过这两种途径沉积的Li 2 O 2的量。系统分析表明,无论体积形态如何,溶液生长的 Li 2 O 2都表现出更高的η RC (> 1 V),这可归因于与表面生长的 Li 2 O 2相比晶体中更高的结构有序度。 据推测,由于电极表面和生长晶体之间的内聚相互作用,表面生长的Li 2 O 2似乎具有微观结构无序,有利于在较低的RC下脱锂诱导部分溶液相氧化(<0 id=32>η不同生长的 Li 2 O 2的RC为对 Li 2 O 2结晶途径的必要控制提供了重要的见解,以提高 Li-O 2电池的能量效率。
更新日期:2020-10-07
中文翻译:

锂氧电池中过氧化锂低能分解途径的定量描述
迫切需要确定非水锂氧(Li-O 2 )电池中过氧化锂(Li 2 O 2 )的低电势分解途径,以改善其较差的能源效率。在这项研究中,实验数据和理论计算表明,Li-O 2电池的再充电过电势( η RC )从根本上取决于Li 2 O 2 结晶途径,而Li 2 O 2结晶途径与放电过程中生长晶体的微观结构特性有着内在的联系。 。通过同时进行表面还原和化学歧化生长的Li 2 O 2似乎形成了两个已解卷积的离散相,并且定量估计了通过这两种途径沉积的Li 2 O 2的量。系统分析表明,无论体积形态如何,溶液生长的 Li 2 O 2都表现出更高的η RC (> 1 V),这可归因于与表面生长的 Li 2 O 2相比晶体中更高的结构有序度。 据推测,由于电极表面和生长晶体之间的内聚相互作用,表面生长的Li 2 O 2似乎具有微观结构无序,有利于在较低的RC下脱锂诱导部分溶液相氧化(<0 id=32>η不同生长的 Li 2 O 2的RC为对 Li 2 O 2结晶途径的必要控制提供了重要的见解,以提高 Li-O 2电池的能量效率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号