Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2020-08-11 , DOI: 10.1016/j.jmb.2020.08.003 Kuldeep Lahry 1 , Aiswarya Gopal 1 , Shivjee Sah 1 , Riyaz Ahmad Shah 1 , Umesh Varshney 2
|
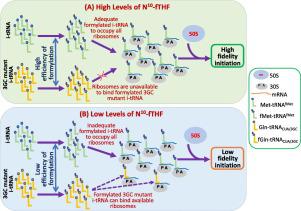
One-carbon metabolism produces methionine and N10-formyl-tetrahydrofolate (N10-fTHF) required for aminoacylation and formylation of initiator tRNA (i-tRNA), respectively. In Escherichia coli, N10-fTHF is made from 5, 10-methylene-THF by a two-step reaction using 5,10-methylene-THF dehydrogenase/cyclohydrolase (FolD). The i-tRNAs from all domains of life possess a highly conserved sequence of three consecutive G–C base pairs (3GC pairs) in their anticodon stem. A 3GC mutant i-tRNA (wherein the 3GC pairs are mutated to those found in elongator tRNAMet) is incompetent in initiation in E. coli (even though it is efficiently aminoacylated and formylated). Here, we show that E. coli strains having mutations in FolD (G122D or C58Y or P140L) allow a plasmid encoded 3GC mutant i-tRNA to participate in initiation. In vitro, the FolD mutants are highly compromised in their dehydrogenase/cyclohydrolase activities leading to reduced production of N10-fTHF and decreased rates of i-tRNA formylation. The perturbation of one-carbon metabolism by trimethoprim (inhibitor of dihydrofolate reductase) phenocopies FolD deficiency and allows initiation with the 3GC mutant i-tRNA. This study reveals an important crosstalk between one-carbon metabolism and the fidelity of translation initiation via formylation of i-tRNA, and suggests that augmentation of the age old sulfa drugs with FolD inhibitors could be an important antibacterial strategy.
中文翻译:

N10-甲酰基四氢叶酸的代谢通量在大肠杆菌的翻译起始保真度中起关键作用。
一碳代谢产生分别用于引发剂tRNA(i-tRNA)的氨基酰化和甲酰化所需的蛋氨酸和N 10-甲酰基-四氢叶酸(N 10 -fTHF)。在大肠杆菌中,使用5,10-亚甲基-THF脱氢酶/环水解酶(FolD)通过两步反应由5,10-亚甲基-THF制得N 10 -fTHF。来自生活所有领域的i-tRNA在其反密码子茎中具有三个连续的G–C碱基对(3GC对)的高度保守序列。3GC突变体i-tRNA(其中3GC对突变为在延伸子tRNA Met中发现的)在大肠杆菌中不能启动(即使它被有效地氨酰化和甲酰化)。在这里,我们表明在FolD中发生突变的大肠杆菌菌株(G122D或C58Y或P140L)允许编码3GC突变体i-tRNA的质粒参与启动。在体外,FolD突变体的脱氢酶/环水解酶活性受到很大损害,导致N 10 -fTHF产量减少和i-tRNA甲酰化速率降低。甲氧苄氨嘧啶(二氢叶酸还原酶抑制剂)的表型会干扰FolD缺乏,并允许3GC突变体i-tRNA引发一碳代谢。这项研究揭示了一个碳代谢与通过i-tRNA甲酰化进行的翻译起始保真度之间的重要相互作用,并表明使用FolD抑制剂来增强古老的磺胺药物可能是重要的抗菌策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号